Interpret minimum inhibitory concentration (MIC) values and disk diffusion diameters according to EUCAST or CLSI, or clean up existing SIR values. This transforms the input to a new class sir, which is an ordered factor with levels S < I < R.
All breakpoints used for interpretation are publicly available in the clinical_breakpoints data set.
Usage
as.sir(x, ...)
NA_sir_
is.sir(x)
is_sir_eligible(x, threshold = 0.05)
# S3 method for mic
as.sir(
x,
mo = NULL,
ab = deparse(substitute(x)),
guideline = getOption("AMR_guideline", "EUCAST"),
uti = NULL,
conserve_capped_values = FALSE,
add_intrinsic_resistance = FALSE,
reference_data = AMR::clinical_breakpoints,
include_screening = getOption("AMR_include_screening", FALSE),
include_PKPD = getOption("AMR_include_PKPD", TRUE),
...
)
# S3 method for disk
as.sir(
x,
mo = NULL,
ab = deparse(substitute(x)),
guideline = getOption("AMR_guideline", "EUCAST"),
uti = NULL,
add_intrinsic_resistance = FALSE,
reference_data = AMR::clinical_breakpoints,
include_screening = getOption("AMR_include_screening", FALSE),
include_PKPD = getOption("AMR_include_PKPD", TRUE),
...
)
# S3 method for data.frame
as.sir(
x,
...,
col_mo = NULL,
guideline = getOption("AMR_guideline", "EUCAST"),
uti = NULL,
conserve_capped_values = FALSE,
add_intrinsic_resistance = FALSE,
reference_data = AMR::clinical_breakpoints,
include_screening = getOption("AMR_include_screening", FALSE),
include_PKPD = getOption("AMR_include_PKPD", TRUE)
)
sir_interpretation_history(clean = FALSE)Source
For interpretations of minimum inhibitory concentration (MIC) values and disk diffusion diameters:
M39 Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data, 2013-2022, Clinical and Laboratory Standards Institute (CLSI). https://clsi.org/standards/products/microbiology/documents/m39/.
M100 Performance Standard for Antimicrobial Susceptibility Testing, 2013-2022, Clinical and Laboratory Standards Institute (CLSI). https://clsi.org/standards/products/microbiology/documents/m100/.
Breakpoint tables for interpretation of MICs and zone diameters, 2013-2022, European Committee on Antimicrobial Susceptibility Testing (EUCAST). https://www.eucast.org/clinical_breakpoints.
Arguments
- x
vector of values (for class
mic: MIC values in mg/L, for classdisk: a disk diffusion radius in millimetres)- ...
for using on a data.frame: names of columns to apply
as.sir()on (supports tidy selection such ascolumn1:column4). Otherwise: arguments passed on to methods.- threshold
maximum fraction of invalid antimicrobial interpretations of
x, see Examples- mo
any (vector of) text that can be coerced to valid microorganism codes with
as.mo(), can be left empty to determine it automatically- ab
any (vector of) text that can be coerced to a valid antimicrobial drug code with
as.ab()- guideline
defaults to EUCAST 2022 (the latest implemented EUCAST guideline in the clinical_breakpoints data set), but can be set with the option
AMR_guideline. Currently supports EUCAST (2013-2022) and CLSI (2013-2022), see Details.- uti
(Urinary Tract Infection) A vector with logicals (
TRUEorFALSE) to specify whether a UTI specific interpretation from the guideline should be chosen. For usingas.sir()on a data.frame, this can also be a column containing logicals or when left blank, the data set will be searched for a column 'specimen', and rows within this column containing 'urin' (such as 'urine', 'urina') will be regarded isolates from a UTI. See Examples.- conserve_capped_values
a logical to indicate that MIC values starting with
">"(but not">=") must always return "R" , and that MIC values starting with"<"(but not"<=") must always return "S"- add_intrinsic_resistance
(only useful when using a EUCAST guideline) a logical to indicate whether intrinsic antibiotic resistance must also be considered for applicable bug-drug combinations, meaning that e.g. ampicillin will always return "R" in Klebsiella species. Determination is based on the intrinsic_resistant data set, that itself is based on 'EUCAST Expert Rules' and 'EUCAST Intrinsic Resistance and Unusual Phenotypes' v3.3 (2021).
- reference_data
a data.frame to be used for interpretation, which defaults to the clinical_breakpoints data set. Changing this argument allows for using own interpretation guidelines. This argument must contain a data set that is equal in structure to the clinical_breakpoints data set (same column names and column types). Please note that the
guidelineargument will be ignored whenreference_datais manually set.- include_screening
a logical to indicate that clinical breakpoints for screening are allowed, defaults to
FALSE. Can also be set with the optionAMR_include_screening.- include_PKPD
a logical to indicate that PK/PD clinical breakpoints must be applied as a last resort, defaults to
TRUE. Can also be set with the optionAMR_include_PKPD.- col_mo
column name of the names or codes of the microorganisms (see
as.mo()), defaults to the first column of classmo. Values will be coerced usingas.mo().- clean
a logical to indicate whether previously stored results should be forgotten after returning the 'logbook' with results
Value
Ordered factor with new class sir
Details
How it Works
The as.sir() function works in four ways:
For cleaning raw / untransformed data. The data will be cleaned to only contain values S, I and R and will try its best to determine this with some intelligence. For example, mixed values with SIR interpretations and MIC values such as
"<0.25; S"will be coerced to"S". Combined interpretations for multiple test methods (as seen in laboratory records) such as"S; S"will be coerced to"S", but a value like"S; I"will returnNAwith a warning that the input is unclear.For interpreting minimum inhibitory concentration (MIC) values according to EUCAST or CLSI. You must clean your MIC values first using
as.mic(), that also gives your columns the new data classmic. Also, be sure to have a column with microorganism names or codes. It will be found automatically, but can be set manually using themoargument.Using
dplyr, SIR interpretation can be done very easily with either:your_data %>% mutate_if(is.mic, as.sir) your_data %>% mutate(across(where(is.mic), as.sir))Operators like "<=" will be stripped before interpretation. When using
conserve_capped_values = TRUE, an MIC value of e.g. ">2" will always return "R", even if the breakpoint according to the chosen guideline is ">=4". This is to prevent that capped values from raw laboratory data would not be treated conservatively. The default behaviour (conserve_capped_values = FALSE) considers ">2" to be lower than ">=4" and might in this case return "S" or "I".
For interpreting disk diffusion diameters according to EUCAST or CLSI. You must clean your disk zones first using
as.disk(), that also gives your columns the new data classdisk. Also, be sure to have a column with microorganism names or codes. It will be found automatically, but can be set manually using themoargument.Using
dplyr, SIR interpretation can be done very easily with either:your_data %>% mutate_if(is.disk, as.sir) your_data %>% mutate(across(where(is.disk), as.sir))
For interpreting a complete data set, with automatic determination of MIC values, disk diffusion diameters, microorganism names or codes, and antimicrobial test results. This is done very simply by running
as.sir(your_data).
For points 2, 3 and 4: Use sir_interpretation_history() to retrieve a data.frame (or tibble if the tibble package is installed) with all results of the last as.sir() call.
Supported Guidelines
For interpreting MIC values as well as disk diffusion diameters, currently implemented guidelines are EUCAST (2013-2022) and CLSI (2013-2022).
Thus, the guideline argument must be set to e.g., "EUCAST 2022" or "CLSI 2022". By simply using "EUCAST" (the default) or "CLSI" as input, the latest included version of that guideline will automatically be selected. You can set your own data set using the reference_data argument. The guideline argument will then be ignored.
You can set the default guideline with the option AMR_guideline (e.g. in your .Rprofile file), such as:
options(AMR_guideline = "CLSI")
options(AMR_guideline = "CLSI 2018")
options(AMR_guideline = "EUCAST 2020")
# or to reset:
options(AMR_guideline = NULL)After Interpretation
After using as.sir(), you can use the eucast_rules() defined by EUCAST to (1) apply inferred susceptibility and resistance based on results of other antimicrobials and (2) apply intrinsic resistance based on taxonomic properties of a microorganism.
Machine-Readable Clinical Breakpoints
The repository of this package contains a machine-readable version of all guidelines. This is a CSV file consisting of 18 308 rows and 11 columns. This file is machine-readable, since it contains one row for every unique combination of the test method (MIC or disk diffusion), the antimicrobial drug and the microorganism. This allows for easy implementation of these rules in laboratory information systems (LIS). Note that it only contains interpretation guidelines for humans - interpretation guidelines from CLSI for animals were removed.
Other
The function is.sir() detects if the input contains class sir. If the input is a data.frame, it iterates over all columns and returns a logical vector.
The function is_sir_eligible() returns TRUE when a columns contains at most 5% invalid antimicrobial interpretations (not S and/or I and/or R), and FALSE otherwise. The threshold of 5% can be set with the threshold argument. If the input is a data.frame, it iterates over all columns and returns a logical vector.
NA_sir_ is a missing value of the new sir class, analogous to e.g. base R's NA_character_.
Interpretation of SIR
In 2019, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) has decided to change the definitions of susceptibility testing categories S, I, and R as shown below (https://www.eucast.org/newsiandr/):
S - Susceptible, standard dosing regimen
A microorganism is categorised as "Susceptible, standard dosing regimen", when there is a high likelihood of therapeutic success using a standard dosing regimen of the agent.I - Susceptible, increased exposure
A microorganism is categorised as "Susceptible, Increased exposure" when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection.R = Resistant
A microorganism is categorised as "Resistant" when there is a high likelihood of therapeutic failure even when there is increased exposure.Exposure is a function of how the mode of administration, dose, dosing interval, infusion time, as well as distribution and excretion of the antimicrobial agent will influence the infecting organism at the site of infection.
This AMR package honours this insight. Use susceptibility() (equal to proportion_SI()) to determine antimicrobial susceptibility and count_susceptible() (equal to count_SI()) to count susceptible isolates.
Reference Data Publicly Available
All data sets in this AMR package (about microorganisms, antibiotics, SIR interpretation, EUCAST rules, etc.) are publicly and freely available for download in the following formats: R, MS Excel, Apache Feather, Apache Parquet, SPSS, SAS, and Stata. We also provide tab-separated plain text files that are machine-readable and suitable for input in any software program, such as laboratory information systems. Please visit our website for the download links. The actual files are of course available on our GitHub repository.
Examples
example_isolates
#> # A tibble: 2,000 × 46
#> date patient age gender ward mo PEN OXA FLC AMX
#> <date> <chr> <dbl> <chr> <chr> <mo> <sir> <sir> <sir> <sir>
#> 1 2002-01-02 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 2 2002-01-03 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 3 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 4 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 5 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 6 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 7 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 8 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 9 2002-01-16 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 10 2002-01-17 858515 79 F ICU B_STPHY_EPDR R NA S NA
#> # … with 1,990 more rows, and 36 more variables: AMC <sir>, AMP <sir>,
#> # TZP <sir>, CZO <sir>, FEP <sir>, CXM <sir>, FOX <sir>, CTX <sir>,
#> # CAZ <sir>, CRO <sir>, GEN <sir>, TOB <sir>, AMK <sir>, KAN <sir>,
#> # TMP <sir>, SXT <sir>, NIT <sir>, FOS <sir>, LNZ <sir>, CIP <sir>,
#> # MFX <sir>, VAN <sir>, TEC <sir>, TCY <sir>, TGC <sir>, DOX <sir>,
#> # ERY <sir>, CLI <sir>, AZM <sir>, IPM <sir>, MEM <sir>, MTR <sir>,
#> # CHL <sir>, COL <sir>, MUP <sir>, RIF <sir>
summary(example_isolates) # see all SIR results at a glance
#> date patient age gender
#> Min. :2002-01-02 Length:2000 Min. : 0.00 Length:2000
#> 1st Qu.:2005-07-31 Class :character 1st Qu.:63.00 Class :character
#> Median :2009-07-31 Mode :character Median :74.00 Mode :character
#> Mean :2009-11-20 Mean :70.69
#> 3rd Qu.:2014-05-30 3rd Qu.:82.00
#> Max. :2017-12-28 Max. :97.00
#> ward mo PEN
#> Length:2000 Class :mo Class:sir
#> Class :character <NA> :0 %R :73.7% (n=1201)
#> Mode :character Unique:90 %SI :26.3% (n=428)
#> #1 :B_ESCHR_COLI - %S :25.6% (n=417)
#> #2 :B_STPHY_CONS - %I : 0.7% (n=11)
#> #3 :B_STPHY_AURS
#> OXA FLC AMX
#> Class:sir Class:sir Class:sir
#> %R :31.2% (n=114) %R :29.5% (n=278) %R :59.6% (n=804)
#> %SI :68.8% (n=251) %SI :70.5% (n=665) %SI :40.4% (n=546)
#> - %S :68.8% (n=251) - %S :70.5% (n=665) - %S :40.2% (n=543)
#> - %I : 0.0% (n=0) - %I : 0.0% (n=0) - %I : 0.2% (n=3)
#>
#> AMC AMP TZP
#> Class:sir Class:sir Class:sir
#> %R :23.7% (n=446) %R :59.6% (n=804) %R :12.6% (n=126)
#> %SI :76.3% (n=1433) %SI :40.4% (n=546) %SI :87.4% (n=875)
#> - %S :71.4% (n=1342) - %S :40.2% (n=543) - %S :86.1% (n=862)
#> - %I : 4.8% (n=91) - %I : 0.2% (n=3) - %I : 1.3% (n=13)
#>
#> CZO FEP CXM
#> Class:sir Class:sir Class:sir
#> %R :44.6% (n=199) %R :14.2% (n=103) %R :26.3% (n=470)
#> %SI :55.4% (n=247) %SI :85.8% (n=621) %SI :73.7% (n=1319)
#> - %S :54.9% (n=245) - %S :85.6% (n=620) - %S :72.5% (n=1297)
#> - %I : 0.4% (n=2) - %I : 0.1% (n=1) - %I : 1.2% (n=22)
#>
#> FOX CTX CAZ
#> Class:sir Class:sir Class:sir
#> %R :27.4% (n=224) %R :15.5% (n=146) %R :66.5% (n=1204)
#> %SI :72.6% (n=594) %SI :84.5% (n=797) %SI :33.5% (n=607)
#> - %S :71.6% (n=586) - %S :84.4% (n=796) - %S :33.5% (n=607)
#> - %I : 1.0% (n=8) - %I : 0.1% (n=1) - %I : 0.0% (n=0)
#>
#> CRO GEN TOB
#> Class:sir Class:sir Class:sir
#> %R :15.5% (n=146) %R :24.6% (n=456) %R :34.4% (n=465)
#> %SI :84.5% (n=797) %SI :75.4% (n=1399) %SI :65.6% (n=886)
#> - %S :84.4% (n=796) - %S :74.0% (n=1372) - %S :65.1% (n=879)
#> - %I : 0.1% (n=1) - %I : 1.5% (n=27) - %I : 0.5% (n=7)
#>
#> AMK KAN TMP
#> Class:sir Class:sir Class:sir
#> %R :63.7% (n=441) %R :100.0% (n=471) %R :38.1% (n=571)
#> %SI :36.3% (n=251) %SI : 0.0% (n=0) %SI :61.9% (n=928)
#> - %S :36.3% (n=251) - %S : 0.0% (n=0) - %S :61.2% (n=918)
#> - %I : 0.0% (n=0) - %I : 0.0% (n=0) - %I : 0.7% (n=10)
#>
#> SXT NIT FOS
#> Class:sir Class:sir Class:sir
#> %R :20.5% (n=361) %R :17.1% (n=127) %R :42.2% (n=148)
#> %SI :79.5% (n=1398) %SI :82.9% (n=616) %SI :57.8% (n=203)
#> - %S :79.1% (n=1392) - %S :76.0% (n=565) - %S :57.8% (n=203)
#> - %I : 0.3% (n=6) - %I : 6.9% (n=51) - %I : 0.0% (n=0)
#>
#> LNZ CIP MFX
#> Class:sir Class:sir Class:sir
#> %R :69.3% (n=709) %R :16.2% (n=228) %R :33.6% (n=71)
#> %SI :30.7% (n=314) %SI :83.8% (n=1181) %SI :66.4% (n=140)
#> - %S :30.7% (n=314) - %S :78.9% (n=1112) - %S :64.5% (n=136)
#> - %I : 0.0% (n=0) - %I : 4.9% (n=69) - %I : 1.9% (n=4)
#>
#> VAN TEC TCY
#> Class:sir Class:sir Class:sir
#> %R :38.3% (n=712) %R :75.7% (n=739) %R :29.8% (n=357)
#> %SI :61.7% (n=1149) %SI :24.3% (n=237) %SI :70.3% (n=843)
#> - %S :61.7% (n=1149) - %S :24.3% (n=237) - %S :68.3% (n=820)
#> - %I : 0.0% (n=0) - %I : 0.0% (n=0) - %I : 1.9% (n=23)
#>
#> TGC DOX ERY
#> Class:sir Class:sir Class:sir
#> %R :12.7% (n=101) %R :27.7% (n=315) %R :57.2% (n=1084)
#> %SI :87.3% (n=697) %SI :72.3% (n=821) %SI :42.8% (n=810)
#> - %S :87.3% (n=697) - %S :71.7% (n=814) - %S :42.3% (n=801)
#> - %I : 0.0% (n=0) - %I : 0.6% (n=7) - %I : 0.5% (n=9)
#>
#> CLI AZM IPM
#> Class:sir Class:sir Class:sir
#> %R :61.2% (n=930) %R :57.2% (n=1084) %R : 6.2% (n=55)
#> %SI :38.8% (n=590) %SI :42.8% (n=810) %SI :93.8% (n=834)
#> - %S :38.6% (n=586) - %S :42.3% (n=801) - %S :92.7% (n=824)
#> - %I : 0.3% (n=4) - %I : 0.5% (n=9) - %I : 1.1% (n=10)
#>
#> MEM MTR CHL
#> Class:sir Class:sir Class:sir
#> %R : 5.9% (n=49) %R :14.7% (n=5) %R :21.4% (n=33)
#> %SI :94.1% (n=780) %SI :85.3% (n=29) %SI :78.6% (n=121)
#> - %S :94.1% (n=780) - %S :85.3% (n=29) - %S :78.6% (n=121)
#> - %I : 0.0% (n=0) - %I : 0.0% (n=0) - %I : 0.0% (n=0)
#>
#> COL MUP RIF
#> Class:sir Class:sir Class:sir
#> %R :81.2% (n=1331) %R : 5.9% (n=16) %R :69.6% (n=698)
#> %SI :18.8% (n=309) %SI :94.1% (n=254) %SI :30.4% (n=305)
#> - %S :18.8% (n=309) - %S :93.0% (n=251) - %S :30.2% (n=303)
#> - %I : 0.0% (n=0) - %I : 1.1% (n=3) - %I : 0.2% (n=2)
#>
# For INTERPRETING disk diffusion and MIC values -----------------------
# a whole data set, even with combined MIC values and disk zones
df <- data.frame(
microorganism = "Escherichia coli",
AMP = as.mic(8),
CIP = as.mic(0.256),
GEN = as.disk(18),
TOB = as.disk(16),
ERY = "R"
)
as.sir(df)
#> => Interpreting MIC values of column 'AMP' (ampicillin) according to EUCAST
#> 2022...
#> OK.
#> => Interpreting MIC values of column 'CIP' (ciprofloxacin) according to
#> EUCAST 2022...
#> OK.
#> => Interpreting disk diffusion zones of column 'GEN' (gentamicin) according
#> to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for gentamicin (GEN) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting disk diffusion zones of column 'TOB' (tobramycin) according
#> to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for tobramycin (TOB) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Assigning class 'sir' to already clean column 'ERY' (erythromycin)...
#> OK.
#> microorganism AMP CIP GEN TOB ERY
#> 1 Escherichia coli S I S S R
# return a 'logbook' about the results:
sir_interpretation_history()
#> # A tibble: 50 × 17
#> datetime index ab_input ab_guid…¹ mo_in…² mo_guideline guide…³
#> <dttm> <int> <chr> <ab> <chr> <mo> <chr>
#> 1 2023-02-12 16:16:37 1 TOB TOB Escher… B_[ORD]_ENTRBCTR EUCAST…
#> 2 2023-02-12 16:16:36 1 GEN GEN Escher… B_[ORD]_ENTRBCTR EUCAST…
#> 3 2023-02-12 16:16:36 1 CIP CIP Escher… B_[ORD]_ENTRBCTR EUCAST…
#> 4 2023-02-12 16:16:36 1 AMP AMP Escher… B_[ORD]_ENTRBCTR EUCAST…
#> 5 2023-02-12 16:16:30 1 CIP CIP B_ESCH… B_[ORD]_ENTRBCTR EUCAST…
#> 6 2023-02-12 16:16:30 2 CIP CIP B_ESCH… B_[ORD]_ENTRBCTR EUCAST…
#> 7 2023-02-12 16:16:30 3 CIP CIP B_ESCH… B_[ORD]_ENTRBCTR EUCAST…
#> 8 2023-02-12 16:16:30 4 CIP CIP B_ESCH… B_[ORD]_ENTRBCTR EUCAST…
#> 9 2023-02-12 16:16:30 5 CIP CIP B_ESCH… B_[ORD]_ENTRBCTR EUCAST…
#> 10 2023-02-12 16:16:30 6 CIP CIP B_ESCH… B_[ORD]_ENTRBCTR EUCAST…
#> # … with 40 more rows, 10 more variables: ref_table <chr>, method <chr>,
#> # input <dbl>, outcome <sir>, breakpoint_S_R <chr>, ab_considered <lgl>,
#> # mo_considered <lgl>, breakpoint_S <lgl>, breakpoint_R <lgl>,
#> # interpretation <lgl>, and abbreviated variable names ¹ab_guideline,
#> # ²mo_input, ³guideline
# for single values
as.sir(
x = as.mic(2),
mo = as.mo("S. pneumoniae"),
ab = "AMP",
guideline = "EUCAST"
)
#> => Interpreting MIC values of 'AMP' (ampicillin) according to EUCAST
#> 2022...
#> Note:
#> • Multiple breakpoints available for ampicillin (AMP) in Streptococcus
#> pneumoniae - assuming body site 'Non-meningitis'.
#> Class 'sir'
#> [1] R
as.sir(
x = as.disk(18),
mo = "Strep pneu", # `mo` will be coerced with as.mo()
ab = "ampicillin", # and `ab` with as.ab()
guideline = "EUCAST"
)
#> => Interpreting disk diffusion zones of 'ampicillin' (AMP) according to
#> EUCAST 2022...
#> OK.
#> Class 'sir'
#> [1] R
# \donttest{
# the dplyr way
if (require("dplyr")) {
df %>% mutate_if(is.mic, as.sir)
df %>% mutate_if(function(x) is.mic(x) | is.disk(x), as.sir)
df %>% mutate(across(where(is.mic), as.sir))
df %>% mutate_at(vars(AMP:TOB), as.sir)
df %>% mutate(across(AMP:TOB, as.sir))
df %>%
mutate_at(vars(AMP:TOB), as.sir, mo = .$microorganism)
# to include information about urinary tract infections (UTI)
data.frame(
mo = "E. coli",
NIT = c("<= 2", 32),
from_the_bladder = c(TRUE, FALSE)
) %>%
as.sir(uti = "from_the_bladder")
data.frame(
mo = "E. coli",
NIT = c("<= 2", 32),
specimen = c("urine", "blood")
) %>%
as.sir() # automatically determines urine isolates
df %>%
mutate_at(vars(AMP:TOB), as.sir, mo = "E. coli", uti = TRUE)
}
#> => Interpreting MIC values of 'AMP' (ampicillin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting MIC values of 'CIP' (ciprofloxacin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting MIC values of 'AMP' (ampicillin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting MIC values of 'CIP' (ciprofloxacin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting disk diffusion zones of 'GEN' (gentamicin) based on column
#> 'microorganism' according to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for gentamicin (GEN) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting disk diffusion zones of 'TOB' (tobramycin) based on column
#> 'microorganism' according to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for tobramycin (TOB) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting MIC values of 'AMP' (ampicillin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting MIC values of 'CIP' (ciprofloxacin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting MIC values of 'AMP' (ampicillin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting MIC values of 'CIP' (ciprofloxacin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting disk diffusion zones of 'GEN' (gentamicin) based on column
#> 'microorganism' according to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for gentamicin (GEN) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting disk diffusion zones of 'TOB' (tobramycin) based on column
#> 'microorganism' according to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for tobramycin (TOB) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting MIC values of 'AMP' (ampicillin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting MIC values of 'CIP' (ciprofloxacin) based on column
#> 'microorganism' according to EUCAST 2022...
#> OK.
#> => Interpreting disk diffusion zones of 'GEN' (gentamicin) based on column
#> 'microorganism' according to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for gentamicin (GEN) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting disk diffusion zones of 'TOB' (tobramycin) based on column
#> 'microorganism' according to EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for tobramycin (TOB) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting MIC values of 'AMP' (ampicillin) according to EUCAST
#> 2022...
#> OK.
#> => Interpreting MIC values of 'CIP' (ciprofloxacin) according to EUCAST
#> 2022...
#> OK.
#> => Interpreting disk diffusion zones of 'GEN' (gentamicin) according to
#> EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for gentamicin (GEN) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting disk diffusion zones of 'TOB' (tobramycin) according to
#> EUCAST 2022...
#> Note:
#> • Breakpoints for UTI and non-UTI available for tobramycin (TOB) in
#> Escherichia coli - assuming non-UTI. Use argument uti to set which
#> isolates are from urine. See ?as.sir.
#> => Interpreting MIC values of column 'NIT' (nitrofurantoin) according to
#> EUCAST 2022...
#> Warning: in as.sir(): interpretation of nitrofurantoin (NIT) is only available for
#> (uncomplicated) urinary tract infections (UTI) for some microorganisms,
#> thus assuming uti = TRUE. See ?as.sir.
#> * WARNING *
#> ℹ Assuming value "urine" in column 'specimen' reflects a urinary tract
#> infection.
#> Use as.sir(uti = FALSE) to prevent this.
#> => Interpreting MIC values of column 'NIT' (nitrofurantoin) according to
#> EUCAST 2022...
#> Warning: in as.sir(): interpretation of nitrofurantoin (NIT) is only available for
#> (uncomplicated) urinary tract infections (UTI) for some microorganisms,
#> thus assuming uti = TRUE. See ?as.sir.
#> * WARNING *
#> => Interpreting MIC values of 'AMP' (ampicillin) according to EUCAST
#> 2022...
#> OK.
#> => Interpreting MIC values of 'CIP' (ciprofloxacin) according to EUCAST
#> 2022...
#> OK.
#> => Interpreting disk diffusion zones of 'GEN' (gentamicin) according to
#> EUCAST 2022...
#> OK.
#> => Interpreting disk diffusion zones of 'TOB' (tobramycin) according to
#> EUCAST 2022...
#> OK.
#> microorganism AMP CIP GEN TOB ERY
#> 1 Escherichia coli S I S S R
# For CLEANING existing SIR values ------------------------------------
as.sir(c("S", "I", "R", "A", "B", "C"))
#> Warning: in as.sir(): 3 results in column '24' truncated (50%) that were invalid
#> antimicrobial interpretations: "A", "B" and "C"
#> Class 'sir'
#> [1] S I R <NA> <NA> <NA>
as.sir("<= 0.002; S") # will return "S"
#> Class 'sir'
#> [1] S
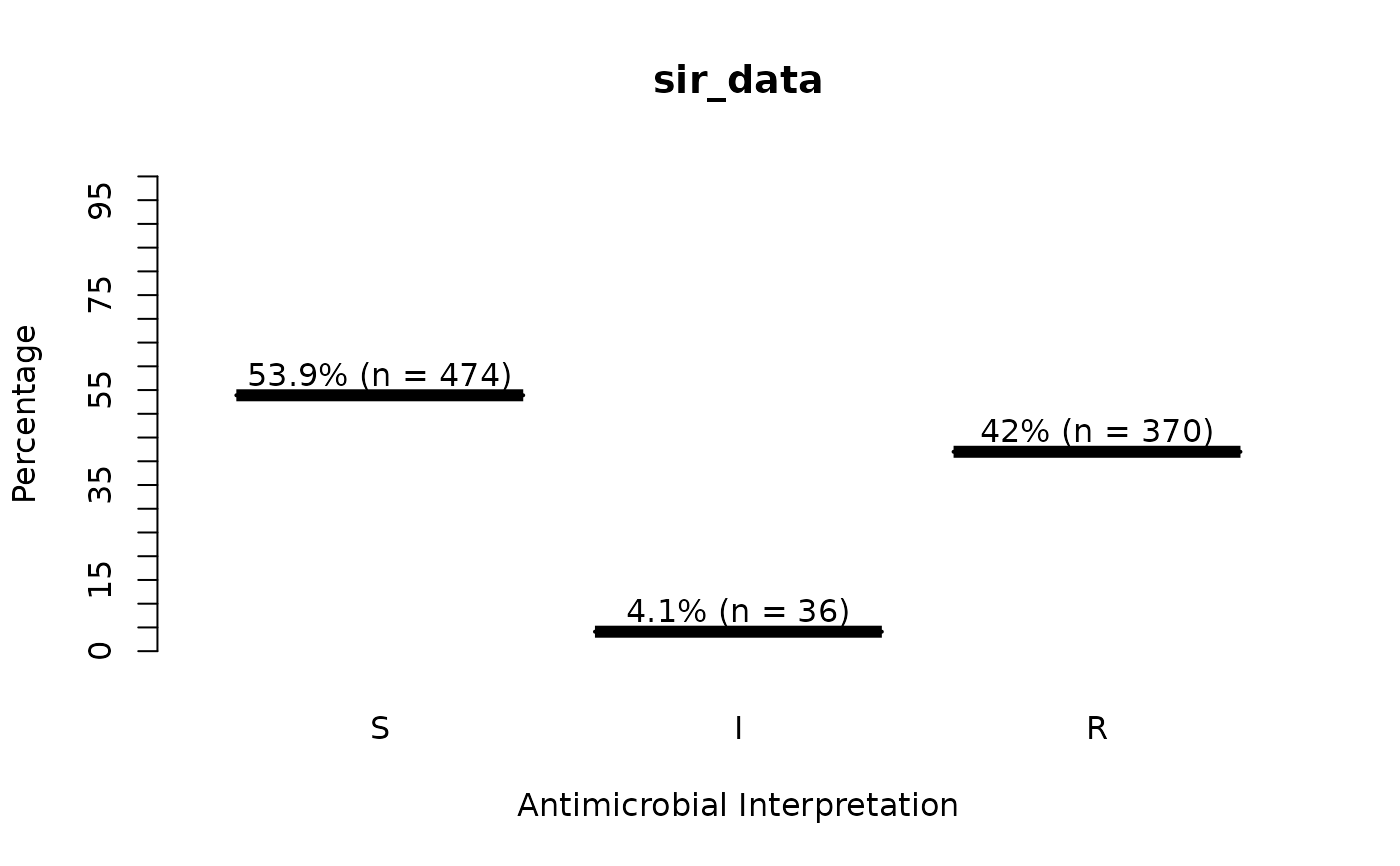
sir_data <- as.sir(c(rep("S", 474), rep("I", 36), rep("R", 370)))
is.sir(sir_data)
#> [1] TRUE
plot(sir_data) # for percentages
 barplot(sir_data) # for frequencies
barplot(sir_data) # for frequencies
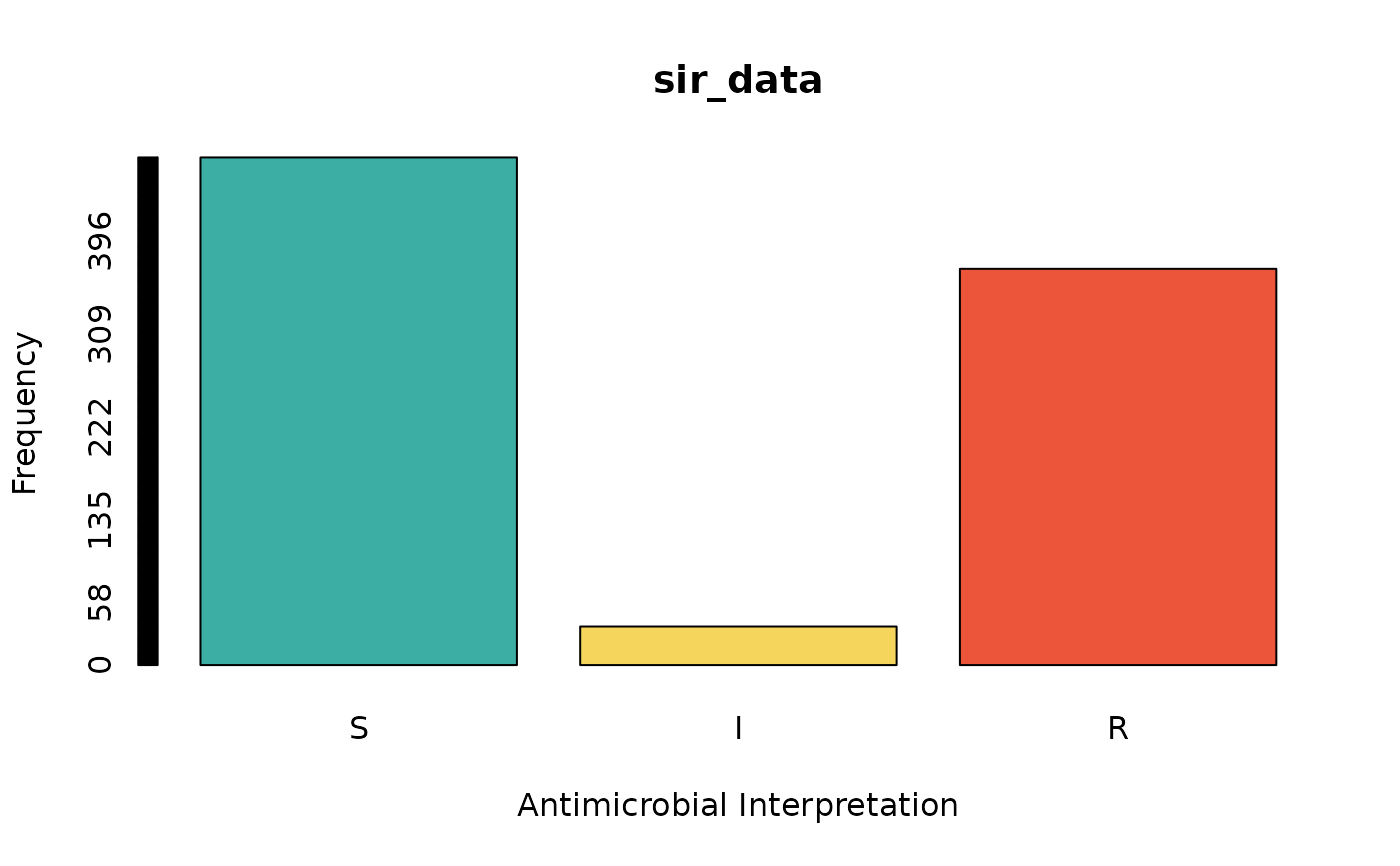 # the dplyr way
if (require("dplyr")) {
example_isolates %>%
mutate_at(vars(PEN:RIF), as.sir)
# same:
example_isolates %>%
as.sir(PEN:RIF)
# fastest way to transform all columns with already valid AMR results to class `sir`:
example_isolates %>%
mutate_if(is_sir_eligible, as.sir)
# since dplyr 1.0.0, this can also be:
# example_isolates %>%
# mutate(across(where(is_sir_eligible), as.sir))
}
#> # A tibble: 2,000 × 46
#> date patient age gender ward mo PEN OXA FLC AMX
#> <date> <chr> <dbl> <chr> <chr> <mo> <sir> <sir> <sir> <sir>
#> 1 2002-01-02 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 2 2002-01-03 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 3 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 4 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 5 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 6 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 7 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 8 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 9 2002-01-16 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 10 2002-01-17 858515 79 F ICU B_STPHY_EPDR R NA S NA
#> # … with 1,990 more rows, and 36 more variables: AMC <sir>, AMP <sir>,
#> # TZP <sir>, CZO <sir>, FEP <sir>, CXM <sir>, FOX <sir>, CTX <sir>,
#> # CAZ <sir>, CRO <sir>, GEN <sir>, TOB <sir>, AMK <sir>, KAN <sir>,
#> # TMP <sir>, SXT <sir>, NIT <sir>, FOS <sir>, LNZ <sir>, CIP <sir>,
#> # MFX <sir>, VAN <sir>, TEC <sir>, TCY <sir>, TGC <sir>, DOX <sir>,
#> # ERY <sir>, CLI <sir>, AZM <sir>, IPM <sir>, MEM <sir>, MTR <sir>,
#> # CHL <sir>, COL <sir>, MUP <sir>, RIF <sir>
# }
# the dplyr way
if (require("dplyr")) {
example_isolates %>%
mutate_at(vars(PEN:RIF), as.sir)
# same:
example_isolates %>%
as.sir(PEN:RIF)
# fastest way to transform all columns with already valid AMR results to class `sir`:
example_isolates %>%
mutate_if(is_sir_eligible, as.sir)
# since dplyr 1.0.0, this can also be:
# example_isolates %>%
# mutate(across(where(is_sir_eligible), as.sir))
}
#> # A tibble: 2,000 × 46
#> date patient age gender ward mo PEN OXA FLC AMX
#> <date> <chr> <dbl> <chr> <chr> <mo> <sir> <sir> <sir> <sir>
#> 1 2002-01-02 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 2 2002-01-03 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 3 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 4 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 5 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 6 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 7 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 8 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 9 2002-01-16 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 10 2002-01-17 858515 79 F ICU B_STPHY_EPDR R NA S NA
#> # … with 1,990 more rows, and 36 more variables: AMC <sir>, AMP <sir>,
#> # TZP <sir>, CZO <sir>, FEP <sir>, CXM <sir>, FOX <sir>, CTX <sir>,
#> # CAZ <sir>, CRO <sir>, GEN <sir>, TOB <sir>, AMK <sir>, KAN <sir>,
#> # TMP <sir>, SXT <sir>, NIT <sir>, FOS <sir>, LNZ <sir>, CIP <sir>,
#> # MFX <sir>, VAN <sir>, TEC <sir>, TCY <sir>, TGC <sir>, DOX <sir>,
#> # ERY <sir>, CLI <sir>, AZM <sir>, IPM <sir>, MEM <sir>, MTR <sir>,
#> # CHL <sir>, COL <sir>, MUP <sir>, RIF <sir>
# }