How to conduct AMR data analysis
Matthijs S. Berends
27 April 2021
Source:vignettes/AMR.Rmd
AMR.RmdNote: values on this page will change with every website update since they are based on randomly created values and the page was written in R Markdown. However, the methodology remains unchanged. This page was generated on 27 April 2021.
Introduction
Conducting AMR data analysis unfortunately requires in-depth knowledge from different scientific fields, which makes it hard to do right. At least, it requires:
- Good questions (always start with those!)
- A thorough understanding of (clinical) epidemiology, to understand the clinical and epidemiological relevance and possible bias of results
- A thorough understanding of (clinical) microbiology/infectious diseases, to understand which microorganisms are causal to which infections and the implications of pharmaceutical treatment, as well as understanding intrinsic and acquired microbial resistance
- Experience with data analysis with microbiological tests and their results, to understand the determination and limitations of MIC values and their interpretations to RSI values
- Availability of the biological taxonomy of microorganisms and probably normalisation factors for pharmaceuticals, such as defined daily doses (DDD)
- Available (inter-)national guidelines, and profound methods to apply them
Of course, we cannot instantly provide you with knowledge and experience. But with this AMR package, we aimed at providing (1) tools to simplify antimicrobial resistance data cleaning, transformation and analysis, (2) methods to easily incorporate international guidelines and (3) scientifically reliable reference data, including the requirements mentioned above.
The AMR package enables standardised and reproducible AMR data analysis, with the application of evidence-based rules, determination of first isolates, translation of various codes for microorganisms and antimicrobial agents, determination of (multi-drug) resistant microorganisms, and calculation of antimicrobial resistance, prevalence and future trends.
Preparation
For this tutorial, we will create fake demonstration data to work with.
You can skip to Cleaning the data if you already have your own data ready. If you start your analysis, try to make the structure of your data generally look like this:
| date | patient_id | mo | AMX | CIP |
|---|---|---|---|---|
| 2021-04-27 | abcd | Escherichia coli | S | S |
| 2021-04-27 | abcd | Escherichia coli | S | R |
| 2021-04-27 | efgh | Escherichia coli | R | S |
Needed R packages
As with many uses in R, we need some additional packages for AMR data analysis. Our package works closely together with the tidyverse packages dplyr and ggplot2 by RStudio. The tidyverse tremendously improves the way we conduct data science - it allows for a very natural way of writing syntaxes and creating beautiful plots in R.
We will also use the cleaner package, that can be used for cleaning data and creating frequency tables.
Creation of data
We will create some fake example data to use for analysis. For AMR data analysis, we need at least: a patient ID, name or code of a microorganism, a date and antimicrobial results (an antibiogram). It could also include a specimen type (e.g. to filter on blood or urine), the ward type (e.g. to filter on ICUs).
With additional columns (like a hospital name, the patients gender of even [well-defined] clinical properties) you can do a comparative analysis, as this tutorial will demonstrate too.
Patients
To start with patients, we need a unique list of patients.
The LETTERS object is available in R - it’s a vector with 26 characters: A to Z. The patients object we just created is now a vector of length 260, with values (patient IDs) varying from A1 to Z10. Now we we also set the gender of our patients, by putting the ID and the gender in a table:
patients_table <- data.frame(patient_id = patients, gender = c(rep("M", 135), rep("F", 125)))
The first 135 patient IDs are now male, the other 125 are female.
Dates
Let’s pretend that our data consists of blood cultures isolates from between 1 January 2010 and 1 January 2018.
This dates object now contains all days in our date range.
Microorganisms
For this tutorial, we will uses four different microorganisms: Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, and Klebsiella pneumoniae:
bacteria <- c("Escherichia coli", "Staphylococcus aureus", "Streptococcus pneumoniae", "Klebsiella pneumoniae")
Put everything together
Using the sample() function, we can randomly select items from all objects we defined earlier. To let our fake data reflect reality a bit, we will also approximately define the probabilities of bacteria and the antibiotic results, using the random_rsi() function.
sample_size <- 20000 data <- data.frame(date = sample(dates, size = sample_size, replace = TRUE), patient_id = sample(patients, size = sample_size, replace = TRUE), hospital = sample(c("Hospital A", "Hospital B", "Hospital C", "Hospital D"), size = sample_size, replace = TRUE, prob = c(0.30, 0.35, 0.15, 0.20)), bacteria = sample(bacteria, size = sample_size, replace = TRUE, prob = c(0.50, 0.25, 0.15, 0.10)), AMX = random_rsi(sample_size, prob_RSI = c(0.35, 0.60, 0.05)), AMC = random_rsi(sample_size, prob_RSI = c(0.15, 0.75, 0.10)), CIP = random_rsi(sample_size, prob_RSI = c(0.20, 0.80, 0.00)), GEN = random_rsi(sample_size, prob_RSI = c(0.08, 0.92, 0.00)))
Using the left_join() function from the dplyr package, we can ‘map’ the gender to the patient ID using the patients_table object we created earlier:
data <- data %>% left_join(patients_table)
The resulting data set contains 20,000 blood culture isolates. With the head() function we can preview the first 6 rows of this data set:
head(data)
| date | patient_id | hospital | bacteria | AMX | AMC | CIP | GEN | gender |
|---|---|---|---|---|---|---|---|---|
| 2016-05-26 | M10 | Hospital D | Staphylococcus aureus | S | S | R | S | M |
| 2014-05-22 | K4 | Hospital B | Staphylococcus aureus | R | S | S | S | M |
| 2012-07-31 | I4 | Hospital D | Streptococcus pneumoniae | R | S | S | S | M |
| 2011-03-22 | V6 | Hospital C | Staphylococcus aureus | S | S | R | S | F |
| 2012-04-09 | G7 | Hospital B | Escherichia coli | I | S | S | S | M |
| 2015-08-25 | Z3 | Hospital C | Staphylococcus aureus | S | S | S | S | F |
Now, let’s start the cleaning and the analysis!
Cleaning the data
We also created a package dedicated to data cleaning and checking, called the cleaner package. It freq() function can be used to create frequency tables.
For example, for the gender variable:
data %>% freq(gender)
Frequency table
Class: character
Length: 20,000
Available: 20,000 (100%, NA: 0 = 0%)
Unique: 2
Shortest: 1
Longest: 1
| Item | Count | Percent | Cum. Count | Cum. Percent | |
|---|---|---|---|---|---|
| 1 | M | 10,477 | 52.39% | 10,477 | 52.39% |
| 2 | F | 9,523 | 47.62% | 20,000 | 100.00% |
So, we can draw at least two conclusions immediately. From a data scientists perspective, the data looks clean: only values M and F. From a researchers perspective: there are slightly more men. Nothing we didn’t already know.
The data is already quite clean, but we still need to transform some variables. The bacteria column now consists of text, and we want to add more variables based on microbial IDs later on. So, we will transform this column to valid IDs. The mutate() function of the dplyr package makes this really easy:
We also want to transform the antibiotics, because in real life data we don’t know if they are really clean. The as.rsi() function ensures reliability and reproducibility in these kind of variables. The mutate_at() will run the as.rsi() function on defined variables:
Finally, we will apply EUCAST rules on our antimicrobial results. In Europe, most medical microbiological laboratories already apply these rules. Our package features their latest insights on intrinsic resistance and exceptional phenotypes. Moreover, the eucast_rules() function can also apply additional rules, like forcing
Because the amoxicillin (column AMX) and amoxicillin/clavulanic acid (column AMC) in our data were generated randomly, some rows will undoubtedly contain AMX = S and AMC = R, which is technically impossible. The eucast_rules() fixes this:
data <- eucast_rules(data, col_mo = "bacteria", rules = "all")
Adding new variables
Now that we have the microbial ID, we can add some taxonomic properties:
data <- data %>% mutate(gramstain = mo_gramstain(bacteria), genus = mo_genus(bacteria), species = mo_species(bacteria))
First isolates
We also need to know which isolates we can actually use for analysis.
To conduct an analysis of antimicrobial resistance, you must only include the first isolate of every patient per episode (Hindler et al., Clin Infect Dis. 2007). If you would not do this, you could easily get an overestimate or underestimate of the resistance of an antibiotic. Imagine that a patient was admitted with an MRSA and that it was found in 5 different blood cultures the following weeks (yes, some countries like the Netherlands have these blood drawing policies). The resistance percentage of oxacillin of all isolates would be overestimated, because you included this MRSA more than once. It would clearly be selection bias.
The Clinical and Laboratory Standards Institute (CLSI) appoints this as follows:
(…) When preparing a cumulative antibiogram to guide clinical decisions about empirical antimicrobial therapy of initial infections, only the first isolate of a given species per patient, per analysis period (eg, one year) should be included, irrespective of body site, antimicrobial susceptibility profile, or other phenotypical characteristics (eg, biotype). The first isolate is easily identified, and cumulative antimicrobial susceptibility test data prepared using the first isolate are generally comparable to cumulative antimicrobial susceptibility test data calculated by other methods, providing duplicate isolates are excluded.
M39-A4 Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data, 4th Edition. CLSI, 2014. Chapter 6.4
This AMR package includes this methodology with the first_isolate() function. It adopts the episode of a year (can be changed by user) and it starts counting days after every selected isolate. This new variable can easily be added to our data:
data <- data %>% mutate(first = first_isolate()) # ℹ Using column 'bacteria' as input for `col_mo`. # ℹ Using column 'date' as input for `col_date`. # ℹ Using column 'patient_id' as input for `col_patient_id`.
So only 53.1% is suitable for resistance analysis! We can now filter on it with the filter() function, also from the dplyr package:
data_1st <- data %>% filter(first == TRUE)
For future use, the above two syntaxes can be shortened with the filter_first_isolate() function:
data_1st <- data %>% filter_first_isolate()
First weighted isolates
We made a slight twist to the CLSI algorithm, to take into account the antimicrobial susceptibility profile. Have a look at all E. coli isolates of patient C8, sorted on date:
| isolate | date | patient_id | bacteria | AMX | AMC | CIP | GEN | first |
|---|---|---|---|---|---|---|---|---|
| 1 | 2010-02-15 | C8 | B_ESCHR_COLI | S | S | S | S | TRUE |
| 2 | 2010-03-23 | C8 | B_ESCHR_COLI | R | S | S | S | FALSE |
| 3 | 2010-05-20 | C8 | B_ESCHR_COLI | S | S | S | S | FALSE |
| 4 | 2011-01-07 | C8 | B_ESCHR_COLI | R | S | S | S | FALSE |
| 5 | 2011-01-09 | C8 | B_ESCHR_COLI | R | S | S | R | FALSE |
| 6 | 2011-01-25 | C8 | B_ESCHR_COLI | S | S | S | S | TRUE |
| 7 | 2011-02-09 | C8 | B_ESCHR_COLI | S | S | R | S | FALSE |
| 8 | 2011-03-09 | C8 | B_ESCHR_COLI | S | S | S | S | TRUE |
| 9 | 2011-04-04 | C8 | B_ESCHR_COLI | R | S | S | S | FALSE |
| 10 | 2011-09-26 | C8 | B_ESCHR_COLI | R | I | S | S | FALSE |
Only 3 isolates are marked as ‘first’ according to CLSI guideline. But when reviewing the antibiogram, it is obvious that some isolates are absolutely different strains and should be included too. This is why we weigh isolates, based on their antibiogram. The key_antibiotics() function adds a vector with 18 key antibiotics: 6 broad spectrum ones, 6 small spectrum for Gram negatives and 6 small spectrum for Gram positives. These can be defined by the user.
If a column exists with a name like ‘key(…)ab’ the first_isolate() function will automatically use it and determine the first weighted isolates. Mind the NOTEs in below output:
data <- data %>% mutate(keyab = key_antibiotics()) %>% mutate(first_weighted = first_isolate())
| isolate | date | patient_id | bacteria | AMX | AMC | CIP | GEN | first | first_weighted |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010-02-15 | C8 | B_ESCHR_COLI | S | S | S | S | TRUE | TRUE |
| 2 | 2010-03-23 | C8 | B_ESCHR_COLI | R | S | S | S | FALSE | FALSE |
| 3 | 2010-05-20 | C8 | B_ESCHR_COLI | S | S | S | S | FALSE | FALSE |
| 4 | 2011-01-07 | C8 | B_ESCHR_COLI | R | S | S | S | FALSE | FALSE |
| 5 | 2011-01-09 | C8 | B_ESCHR_COLI | R | S | S | R | FALSE | FALSE |
| 6 | 2011-01-25 | C8 | B_ESCHR_COLI | S | S | S | S | TRUE | TRUE |
| 7 | 2011-02-09 | C8 | B_ESCHR_COLI | S | S | R | S | FALSE | FALSE |
| 8 | 2011-03-09 | C8 | B_ESCHR_COLI | S | S | S | S | TRUE | TRUE |
| 9 | 2011-04-04 | C8 | B_ESCHR_COLI | R | S | S | S | FALSE | FALSE |
| 10 | 2011-09-26 | C8 | B_ESCHR_COLI | R | I | S | S | FALSE | FALSE |
Instead of 3, now 3 isolates are flagged. In total, 53.1% of all isolates are marked ‘first weighted’ - 0% more than when using the CLSI guideline. In real life, this novel algorithm will yield 5-10% more isolates than the classic CLSI guideline.
As with filter_first_isolate(), there’s a shortcut for this new algorithm too:
data_1st <- data %>% filter_first_weighted_isolate()
So we end up with 10,622 isolates for analysis.
We can remove unneeded columns:
Now our data looks like:
head(data_1st)
| date | patient_id | hospital | bacteria | AMX | AMC | CIP | GEN | gender | gramstain | genus | species | first_weighted | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2014-05-22 | K4 | Hospital B | B_STPHY_AURS | R | S | S | S | M | Gram-positive | Staphylococcus | aureus | TRUE |
| 3 | 2012-07-31 | I4 | Hospital D | B_STRPT_PNMN | R | R | S | R | M | Gram-positive | Streptococcus | pneumoniae | TRUE |
| 5 | 2012-04-09 | G7 | Hospital B | B_ESCHR_COLI | I | S | S | S | M | Gram-negative | Escherichia | coli | TRUE |
| 9 | 2010-04-07 | K8 | Hospital B | B_ESCHR_COLI | R | S | S | S | M | Gram-negative | Escherichia | coli | TRUE |
| 11 | 2015-04-22 | U6 | Hospital D | B_ESCHR_COLI | R | R | R | S | F | Gram-negative | Escherichia | coli | TRUE |
| 14 | 2012-10-27 | U5 | Hospital A | B_KLBSL_PNMN | R | S | R | S | F | Gram-negative | Klebsiella | pneumoniae | TRUE |
Time for the analysis!
Analysing the data
You might want to start by getting an idea of how the data is distributed. It’s an important start, because it also decides how you will continue your analysis. Although this package contains a convenient function to make frequency tables, exploratory data analysis (EDA) is not the primary scope of this package. Use a package like DataExplorer for that, or read the free online book Exploratory Data Analysis with R by Roger D. Peng.
Dispersion of species
To just get an idea how the species are distributed, create a frequency table with our freq() function. We created the genus and species column earlier based on the microbial ID. With paste(), we can concatenate them together.
The freq() function can be used like the base R language was intended:
Or can be used like the dplyr way, which is easier readable:
data_1st %>% freq(genus, species)
Frequency table
Class: character
Length: 10,622
Available: 10,622 (100%, NA: 0 = 0%)
Unique: 4
Shortest: 16
Longest: 24
| Item | Count | Percent | Cum. Count | Cum. Percent | |
|---|---|---|---|---|---|
| 1 | Escherichia coli | 4,606 | 43.36% | 4,606 | 43.36% |
| 2 | Staphylococcus aureus | 2,710 | 25.51% | 7,316 | 68.88% |
| 3 | Streptococcus pneumoniae | 2,131 | 20.06% | 9,447 | 88.94% |
| 4 | Klebsiella pneumoniae | 1,175 | 11.06% | 10,622 | 100.00% |
Overview of different bug/drug combinations
If you want to get a quick glance of the number of isolates in different bug/drug combinations, you can use the bug_drug_combinations() function:
data_1st %>% bug_drug_combinations() %>% head() # show first 6 rows
| mo | ab | S | I | R | total |
|---|---|---|---|---|---|
| E. coli | AMX | 2158 | 150 | 2298 | 4606 |
| E. coli | AMC | 3349 | 191 | 1066 | 4606 |
| E. coli | CIP | 3327 | 0 | 1279 | 4606 |
| E. coli | GEN | 4059 | 0 | 547 | 4606 |
| K. pneumoniae | AMX | 0 | 0 | 1175 | 1175 |
| K. pneumoniae | AMC | 927 | 46 | 202 | 1175 |
Using Tidyverse selections, you can also select columns based on the antibiotic class they are in:
data_1st %>% select(bacteria, fluoroquinolones()) %>% bug_drug_combinations()
# Selecting fluoroquinolones: column 'CIP' (ciprofloxacin)| mo | ab | S | I | R | total |
|---|---|---|---|---|---|
| E. coli | CIP | 3327 | 0 | 1279 | 4606 |
| K. pneumoniae | CIP | 917 | 0 | 258 | 1175 |
| S. aureus | CIP | 2035 | 0 | 675 | 2710 |
| S. pneumoniae | CIP | 1698 | 0 | 433 | 2131 |
This will only give you the crude numbers in the data. To calculate antimicrobial resistance, we use the resistance() and susceptibility() functions.
Resistance percentages
The functions resistance() and susceptibility() can be used to calculate antimicrobial resistance or susceptibility. For more specific analyses, the functions proportion_S(), proportion_SI(), proportion_I(), proportion_IR() and proportion_R() can be used to determine the proportion of a specific antimicrobial outcome.
As per the EUCAST guideline of 2019, we calculate resistance as the proportion of R (proportion_R(), equal to resistance()) and susceptibility as the proportion of S and I (proportion_SI(), equal to susceptibility()). These functions can be used on their own:
data_1st %>% resistance(AMX) # [1] 0.5462248
Or can be used in conjuction with group_by() and summarise(), both from the dplyr package:
data_1st %>% group_by(hospital) %>% summarise(amoxicillin = resistance(AMX))
# `summarise()` ungrouping output (override with `.groups` argument)| hospital | amoxicillin |
|---|---|
| Hospital A | 0.5469388 |
| Hospital B | 0.5456022 |
| Hospital C | 0.5451654 |
| Hospital D | 0.5470046 |
Of course it would be very convenient to know the number of isolates responsible for the percentages. For that purpose the n_rsi() can be used, which works exactly like n_distinct() from the dplyr package. It counts all isolates available for every group (i.e. values S, I or R):
data_1st %>% group_by(hospital) %>% summarise(amoxicillin = resistance(AMX), available = n_rsi(AMX))
# `summarise()` ungrouping output (override with `.groups` argument)| hospital | amoxicillin | available |
|---|---|---|
| Hospital A | 0.5469388 | 3185 |
| Hospital B | 0.5456022 | 3695 |
| Hospital C | 0.5451654 | 1572 |
| Hospital D | 0.5470046 | 2170 |
These functions can also be used to get the proportion of multiple antibiotics, to calculate empiric susceptibility of combination therapies very easily:
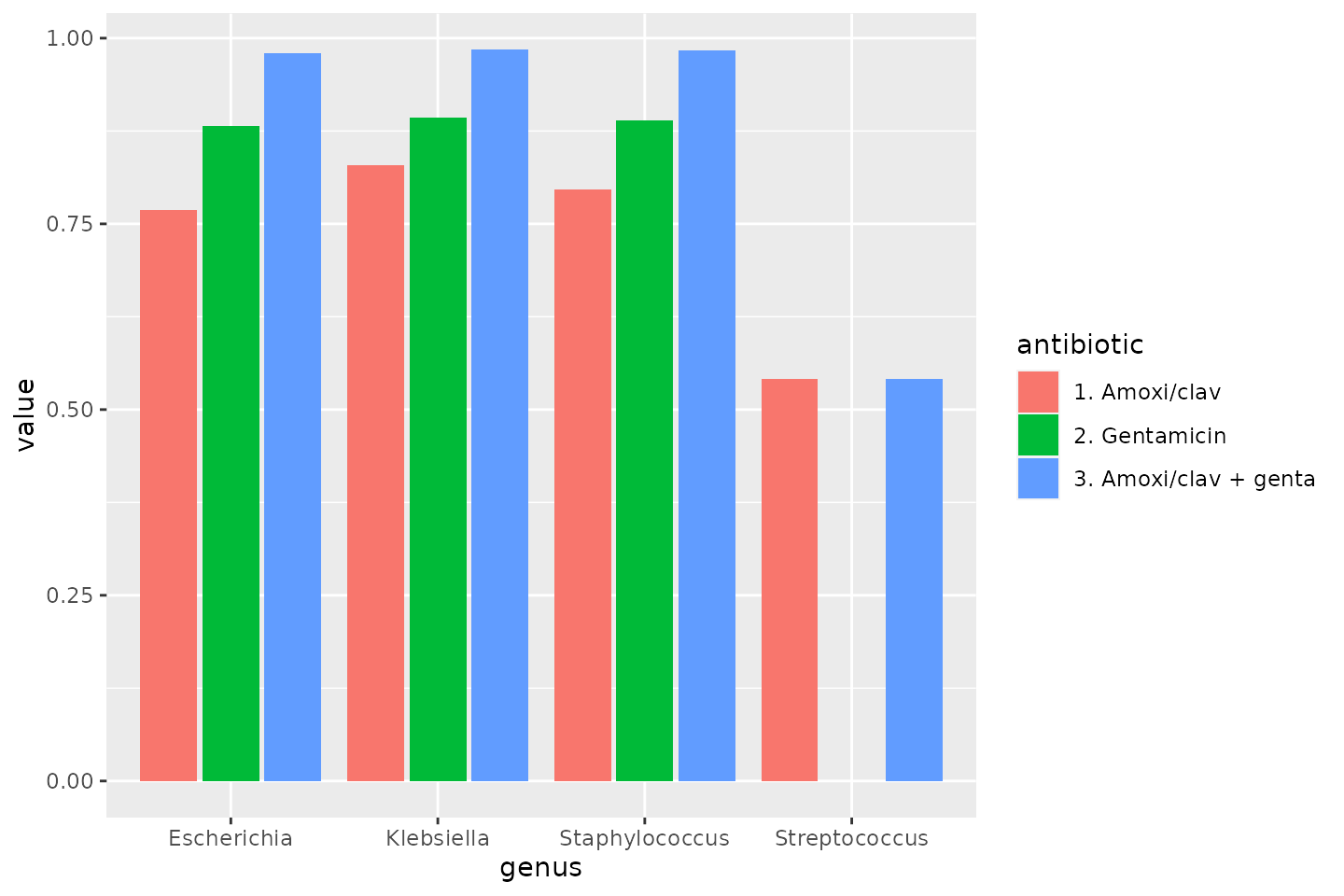
data_1st %>% group_by(genus) %>% summarise(amoxiclav = susceptibility(AMC), gentamicin = susceptibility(GEN), amoxiclav_genta = susceptibility(AMC, GEN))
# `summarise()` ungrouping output (override with `.groups` argument)| genus | amoxiclav | gentamicin | amoxiclav_genta |
|---|---|---|---|
| Escherichia | 0.7685627 | 0.8812419 | 0.9789405 |
| Klebsiella | 0.8280851 | 0.8919149 | 0.9838298 |
| Staphylococcus | 0.7952030 | 0.8892989 | 0.9826568 |
| Streptococcus | 0.5405913 | 0.0000000 | 0.5405913 |
To make a transition to the next part, let’s see how this difference could be plotted:
data_1st %>% group_by(genus) %>% summarise("1. Amoxi/clav" = susceptibility(AMC), "2. Gentamicin" = susceptibility(GEN), "3. Amoxi/clav + genta" = susceptibility(AMC, GEN)) %>% # pivot_longer() from the tidyr package "lengthens" data: tidyr::pivot_longer(-genus, names_to = "antibiotic") %>% ggplot(aes(x = genus, y = value, fill = antibiotic)) + geom_col(position = "dodge2") # `summarise()` ungrouping output (override with `.groups` argument)
Plots
To show results in plots, most R users would nowadays use the ggplot2 package. This package lets you create plots in layers. You can read more about it on their website. A quick example would look like these syntaxes:
ggplot(data = a_data_set, mapping = aes(x = year, y = value)) + geom_col() + labs(title = "A title", subtitle = "A subtitle", x = "My X axis", y = "My Y axis") # or as short as: ggplot(a_data_set) + geom_bar(aes(year))
The AMR package contains functions to extend this ggplot2 package, for example geom_rsi(). It automatically transforms data with count_df() or proportion_df() and show results in stacked bars. Its simplest and shortest example:
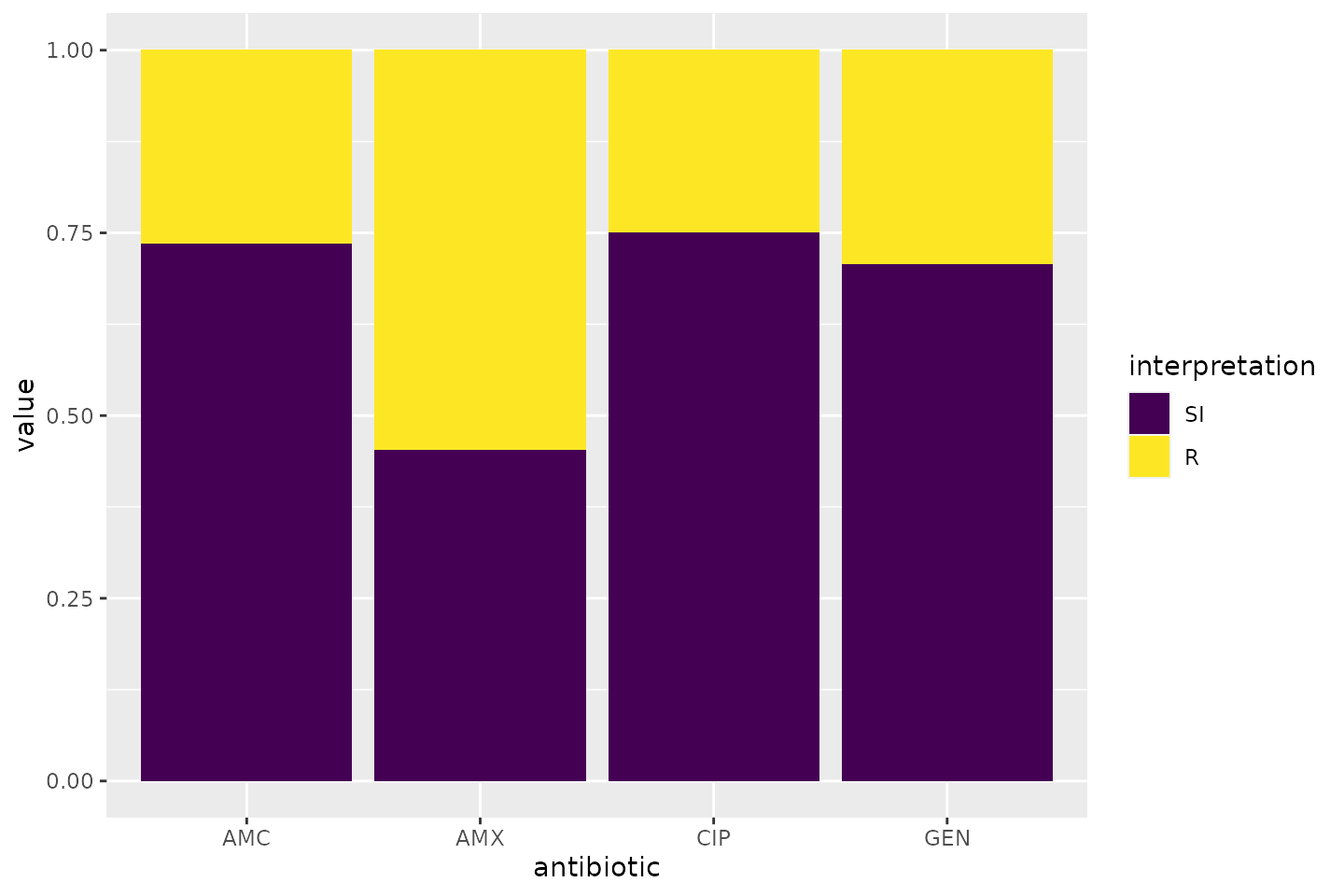
Omit the translate_ab = FALSE to have the antibiotic codes (AMX, AMC, CIP, GEN) translated to official WHO names (amoxicillin, amoxicillin/clavulanic acid, ciprofloxacin, gentamicin).
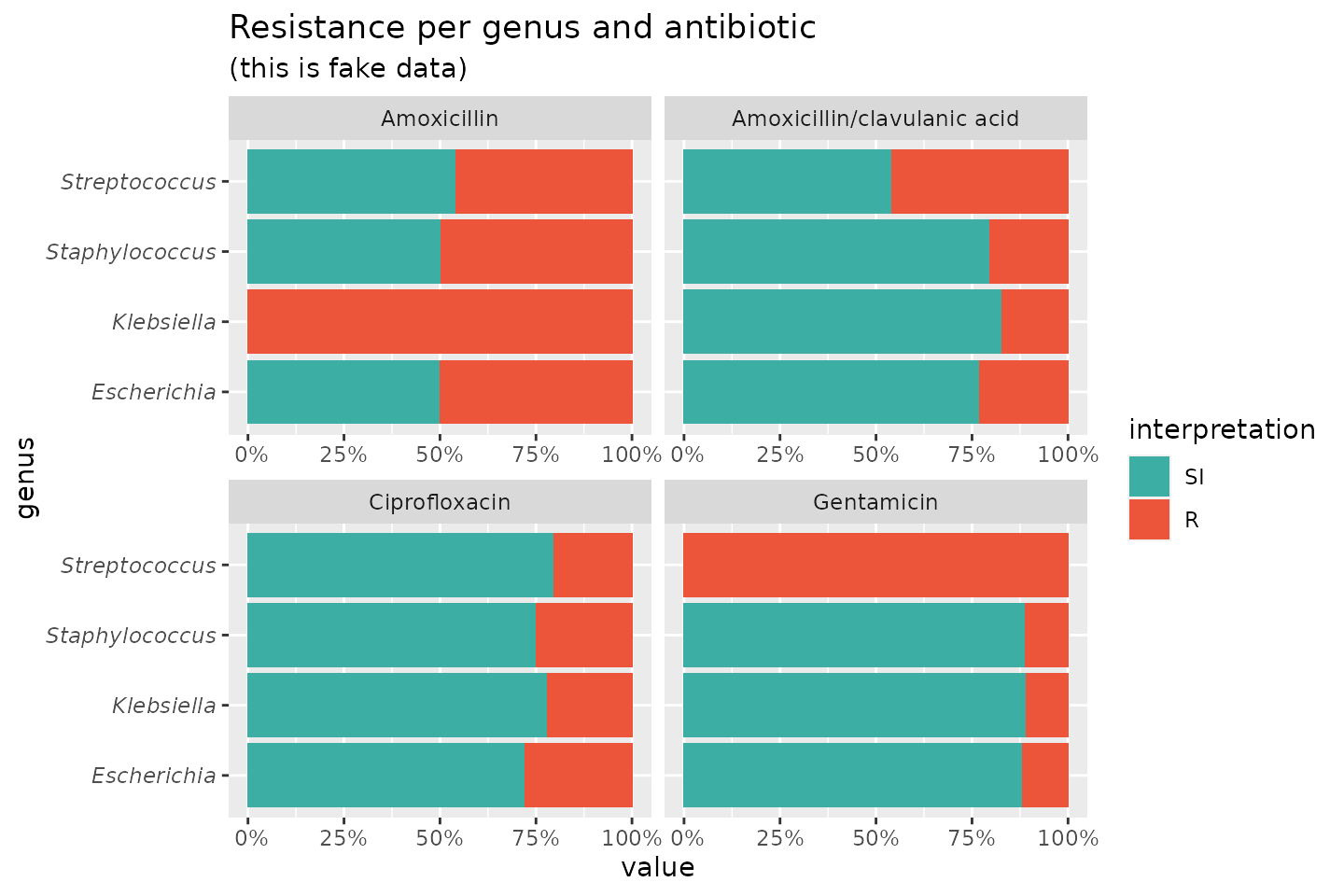
If we group on e.g. the genus column and add some additional functions from our package, we can create this:
# group the data on `genus` ggplot(data_1st %>% group_by(genus)) + # create bars with genus on x axis # it looks for variables with class `rsi`, # of which we have 4 (earlier created with `as.rsi`) geom_rsi(x = "genus") + # split plots on antibiotic facet_rsi(facet = "antibiotic") + # set colours to the R/SI interpretations (colour-blind friendly) scale_rsi_colours() + # show percentages on y axis scale_y_percent(breaks = 0:4 * 25) + # turn 90 degrees, to make it bars instead of columns coord_flip() + # add labels labs(title = "Resistance per genus and antibiotic", subtitle = "(this is fake data)") + # and print genus in italic to follow our convention # (is now y axis because we turned the plot) theme(axis.text.y = element_text(face = "italic"))
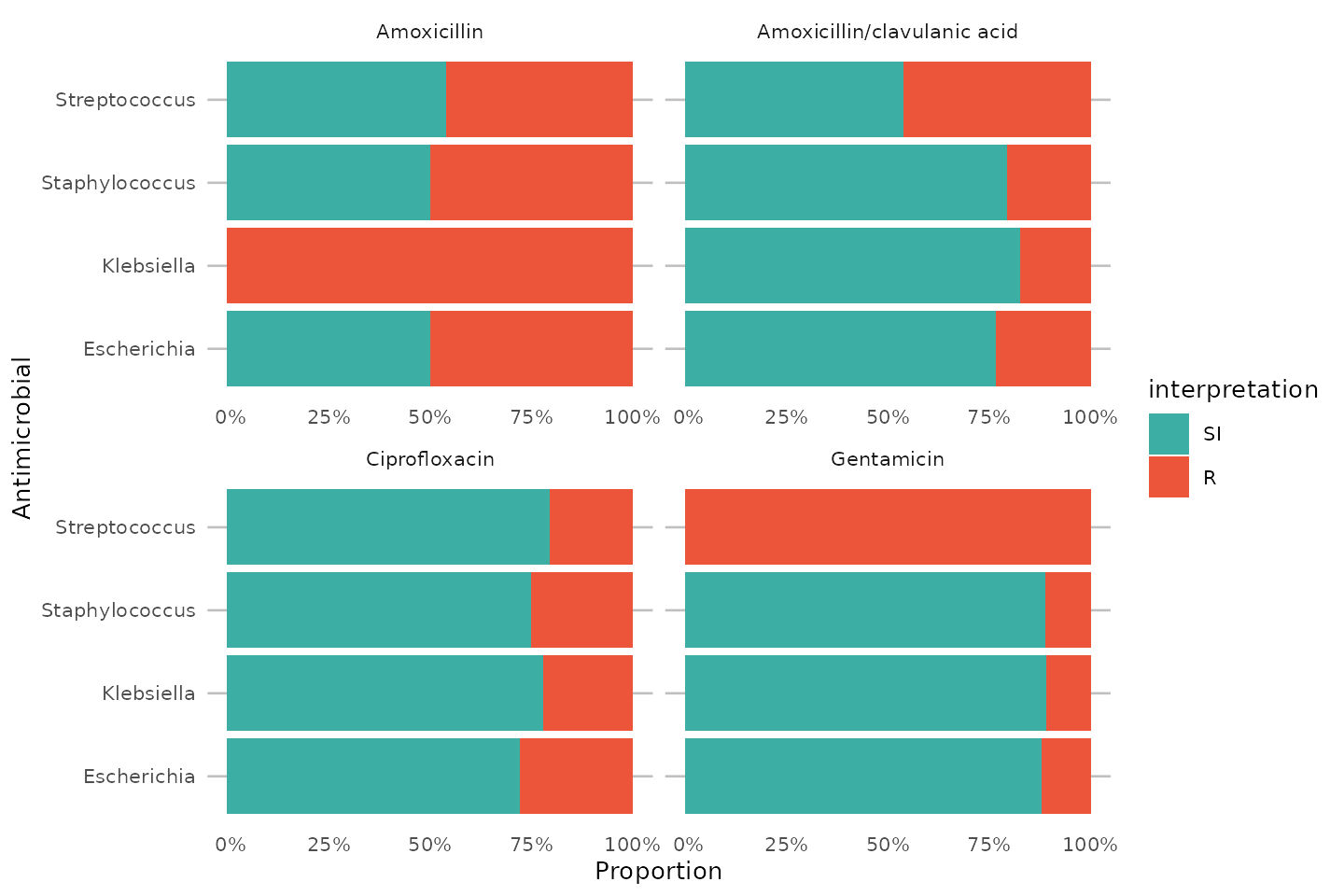
To simplify this, we also created the ggplot_rsi() function, which combines almost all above functions:
data_1st %>% group_by(genus) %>% ggplot_rsi(x = "genus", facet = "antibiotic", breaks = 0:4 * 25, datalabels = FALSE) + coord_flip()
Plotting MIC and disk diffusion values
The AMR package also extends the plot() and ggplot() functions for plotting minimum inhibitory concentrations (MIC, created with as.mic()) and disk diffusion diameters (created with as.disk()).
With the random_mic() and random_disk() functions, we can generate sampled values for the new data types (S3 classes) <mic> and <disk>:
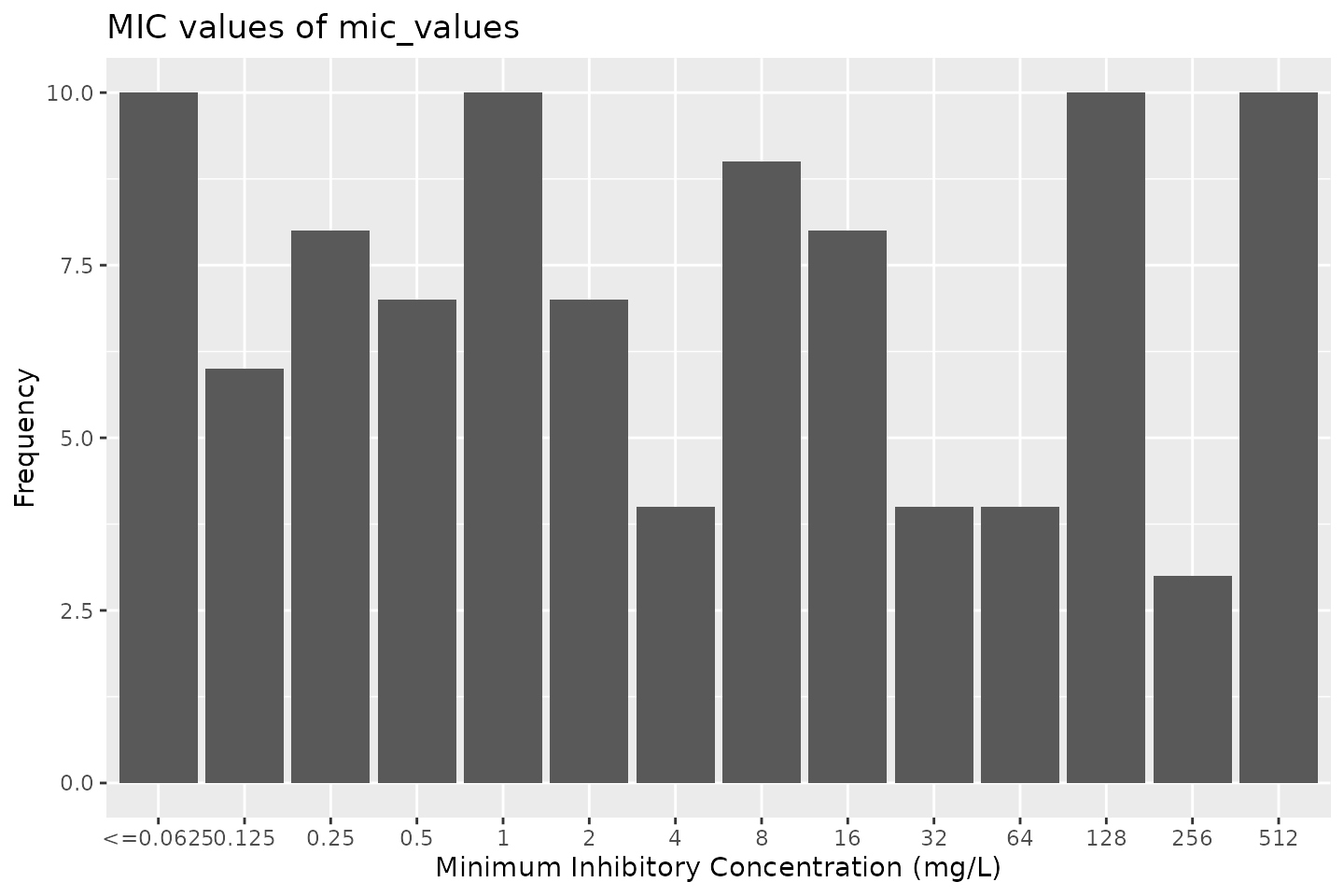
mic_values <- random_mic(size = 100) mic_values # Class <mic> # [1] 16 512 256 16 512 1 256 512 # [9] 2 16 1 16 <=0.0625 1 0.5 <=0.0625 # [17] 8 128 16 0.125 0.125 1 1 4 # [25] 0.5 0.125 8 128 2 <=0.0625 <=0.0625 128 # [33] 0.5 64 512 0.25 32 32 1 4 # [41] 4 256 32 1 1 0.5 512 128 # [49] 0.125 0.25 8 128 8 8 <=0.0625 0.5 # [57] 8 2 64 0.25 8 16 512 4 # [65] 2 512 0.25 0.5 8 128 2 512 # [73] 16 1 128 2 512 32 <=0.0625 0.125 # [81] 128 16 512 0.125 64 128 0.25 1 # [89] 0.25 64 <=0.0625 0.25 128 2 0.5 8 # [97] <=0.0625 <=0.0625 <=0.0625 0.25
# base R: plot(mic_values)
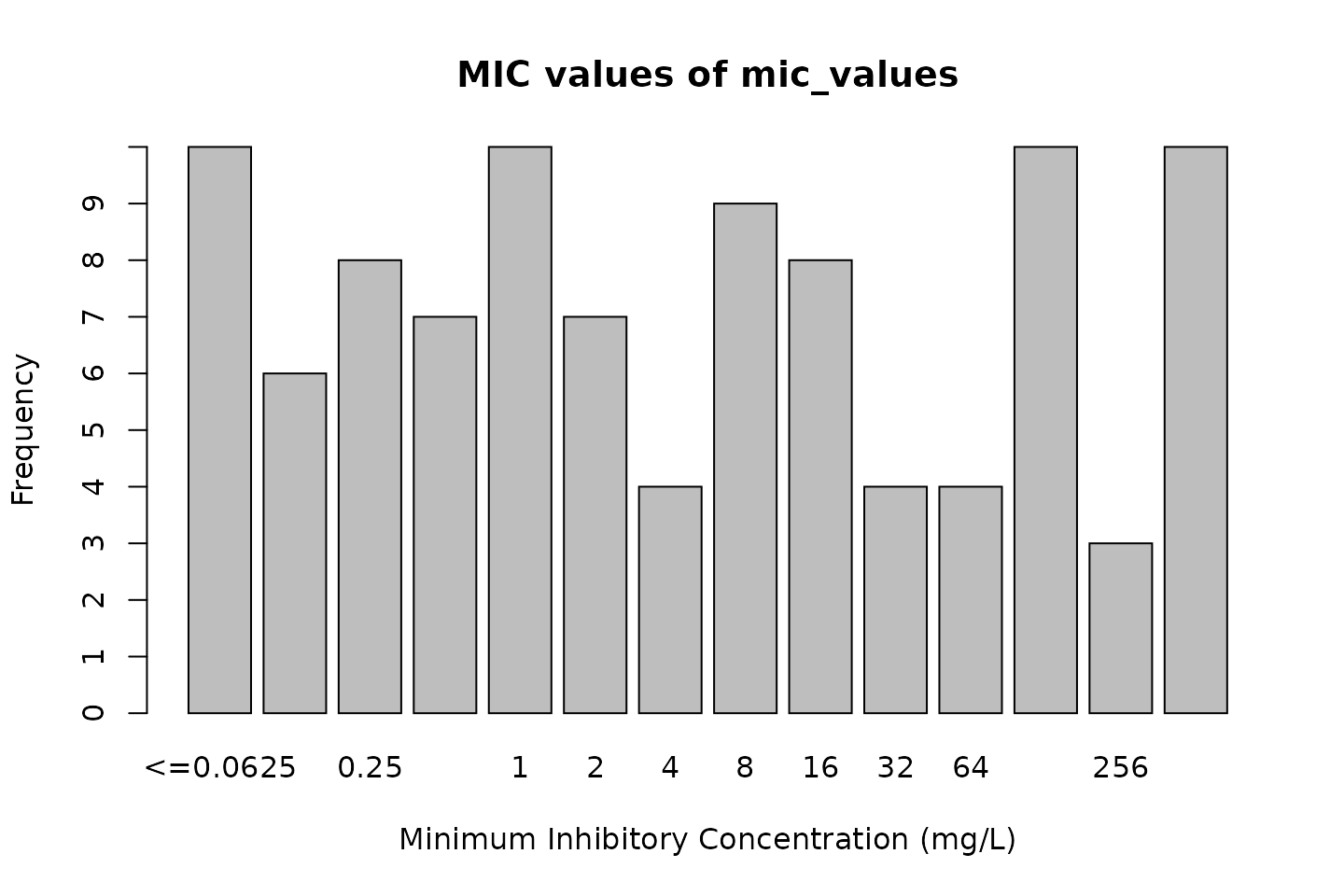
# ggplot2: ggplot(mic_values)
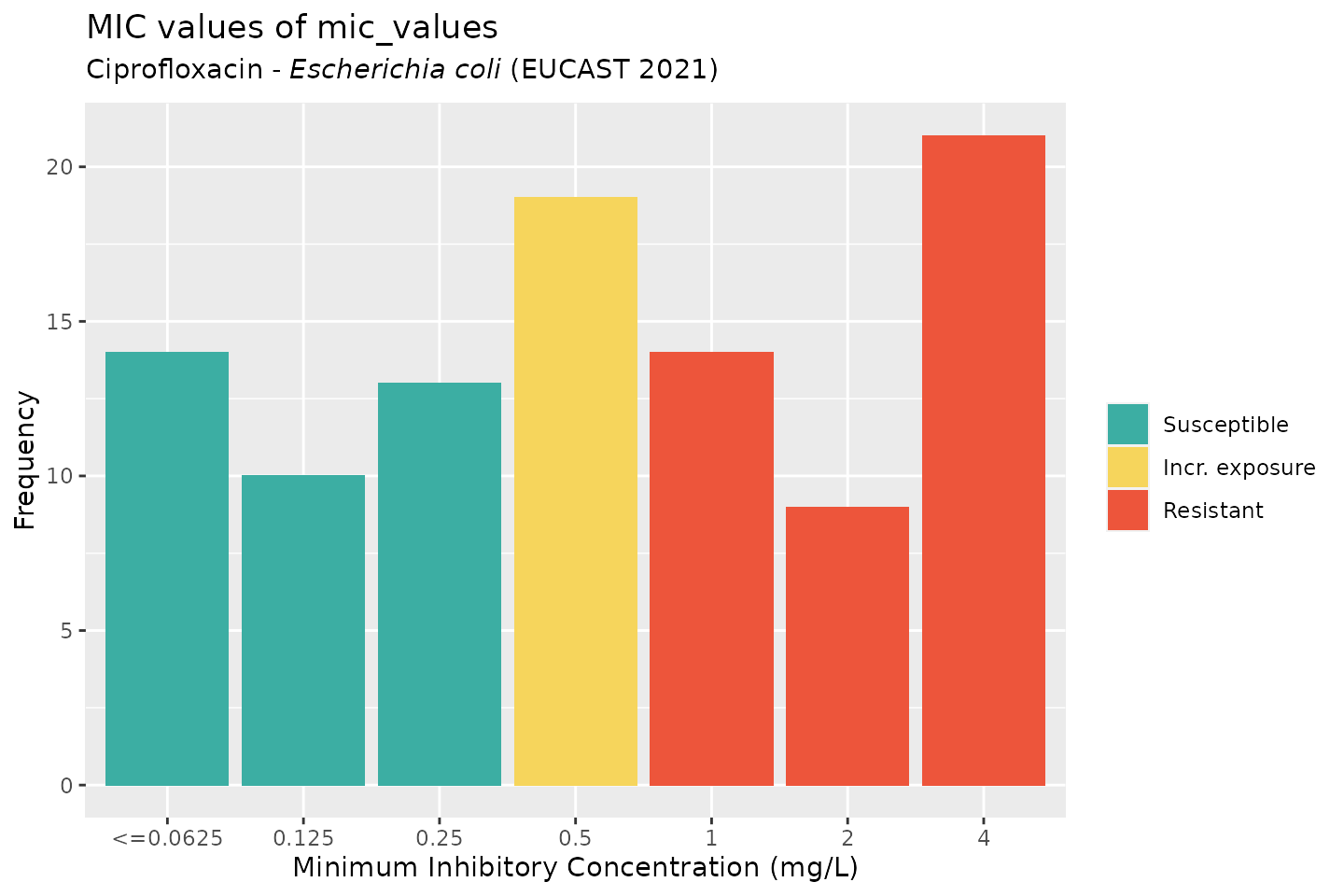
But we could also be more specific, by generating MICs that are likely to be found in E. coli for ciprofloxacin:
mic_values <- random_mic(size = 100, mo = "E. coli", ab = "cipro")
For the plot() and ggplot() function, we can define the microorganism and an antimicrobial agent the same way. This will add the interpretation of those values according to a chosen guidelines (defaults to the latest EUCAST guideline).
Default colours are colour-blind friendly, while maintaining the convention that e.g. ‘susceptible’ should be green and ‘resistant’ should be red:
# base R: plot(mic_values, mo = "E. coli", ab = "cipro")
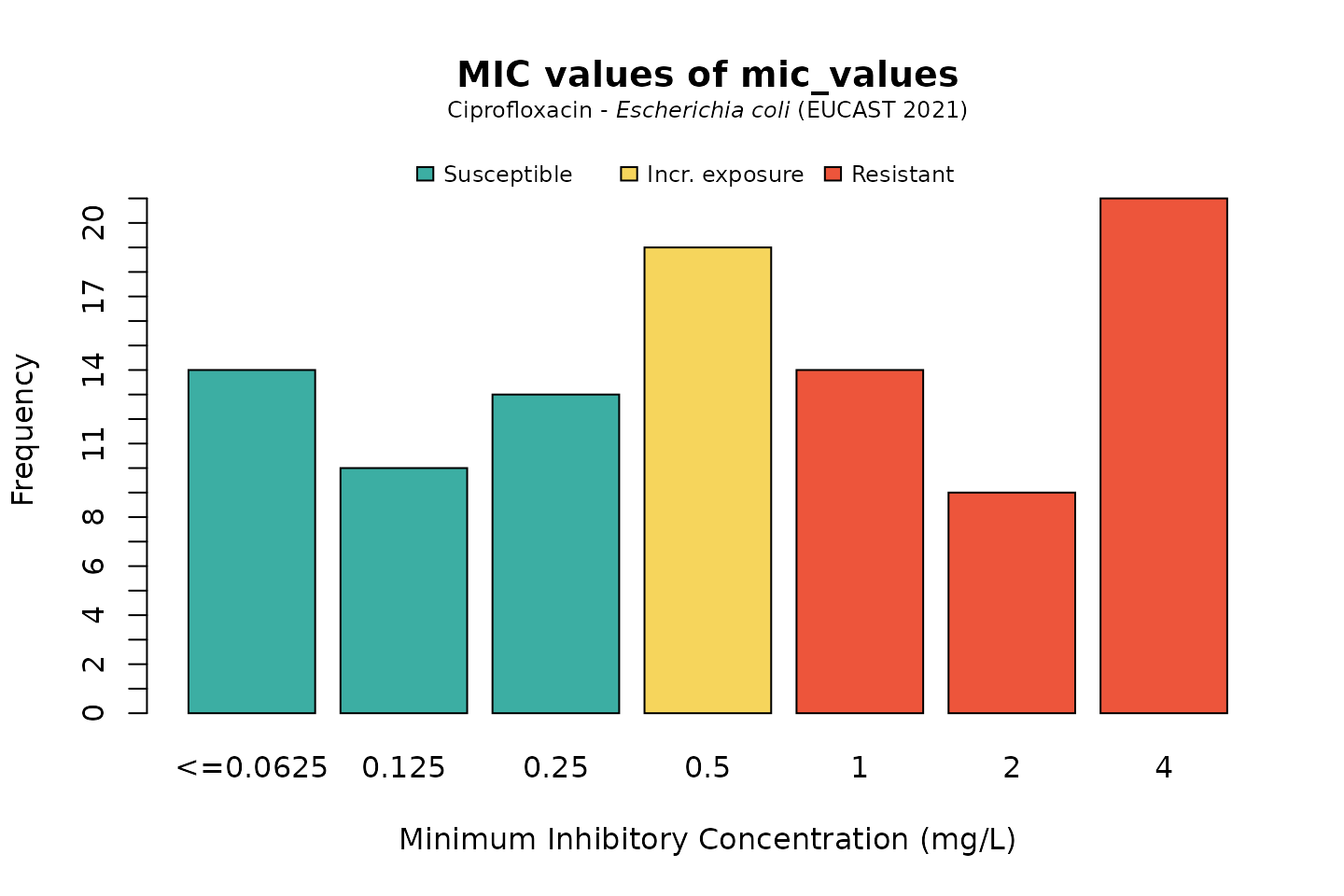
# ggplot2: ggplot(mic_values, mo = "E. coli", ab = "cipro")
For disk diffusion values, there is not much of a difference in plotting:
disk_values <- random_disk(size = 100, mo = "E. coli", ab = "cipro") # ℹ Translation is uncertain of one microorganism. Use `mo_uncertainties()` # to review it. disk_values # Class <disk> # [1] 25 22 17 30 26 27 24 22 30 23 31 26 21 29 19 27 27 23 22 18 26 30 28 30 28 # [26] 18 20 22 20 22 21 22 24 22 21 25 18 31 26 18 21 24 31 17 22 17 31 19 31 25 # [51] 19 21 28 24 18 27 23 19 26 26 20 18 19 30 23 20 19 30 24 27 21 17 31 30 23 # [76] 19 29 23 27 28 21 17 27 20 23 28 31 28 29 31 27 24 18 19 29 31 18 29 17 28
# base R: plot(disk_values, mo = "E. coli", ab = "cipro")
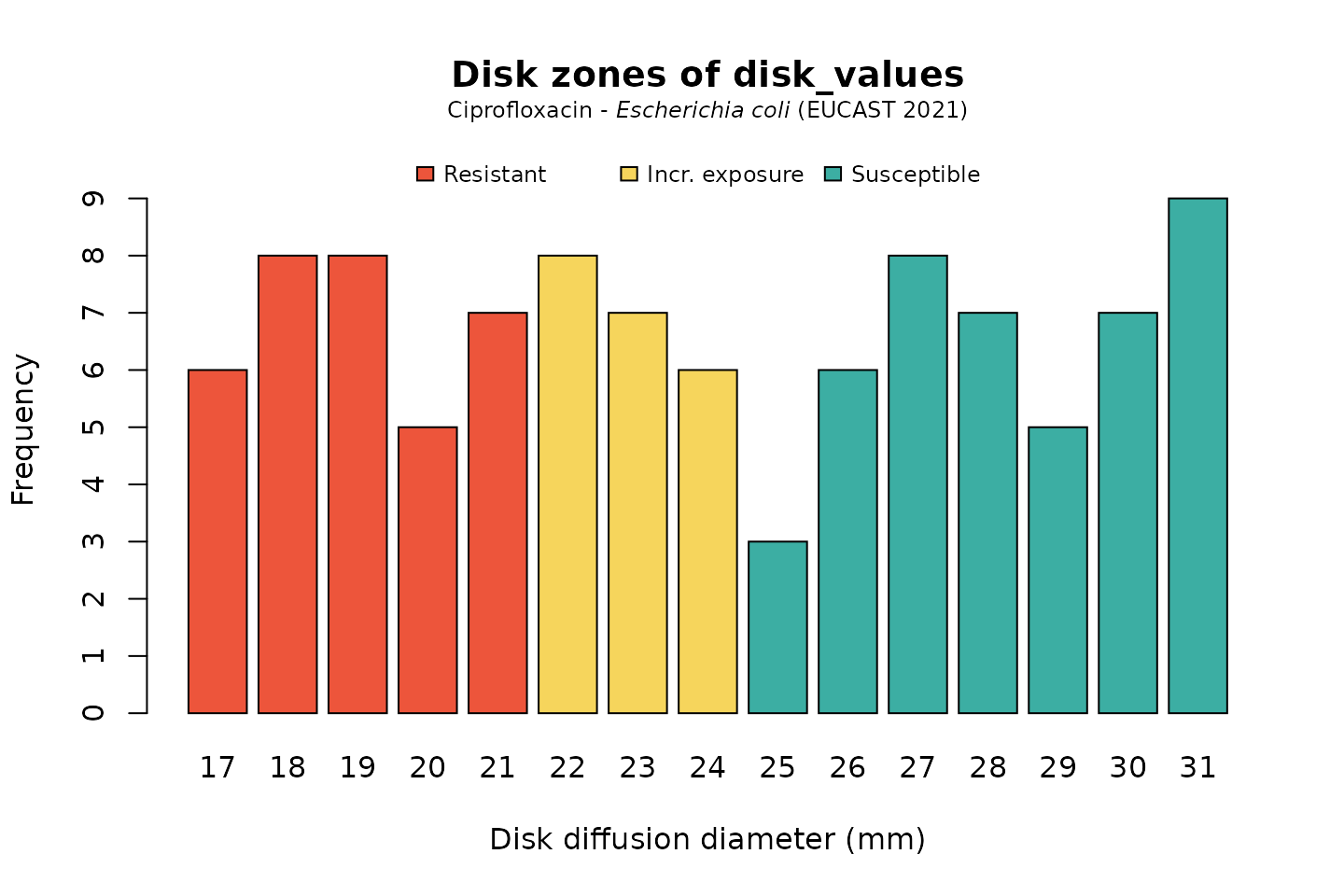
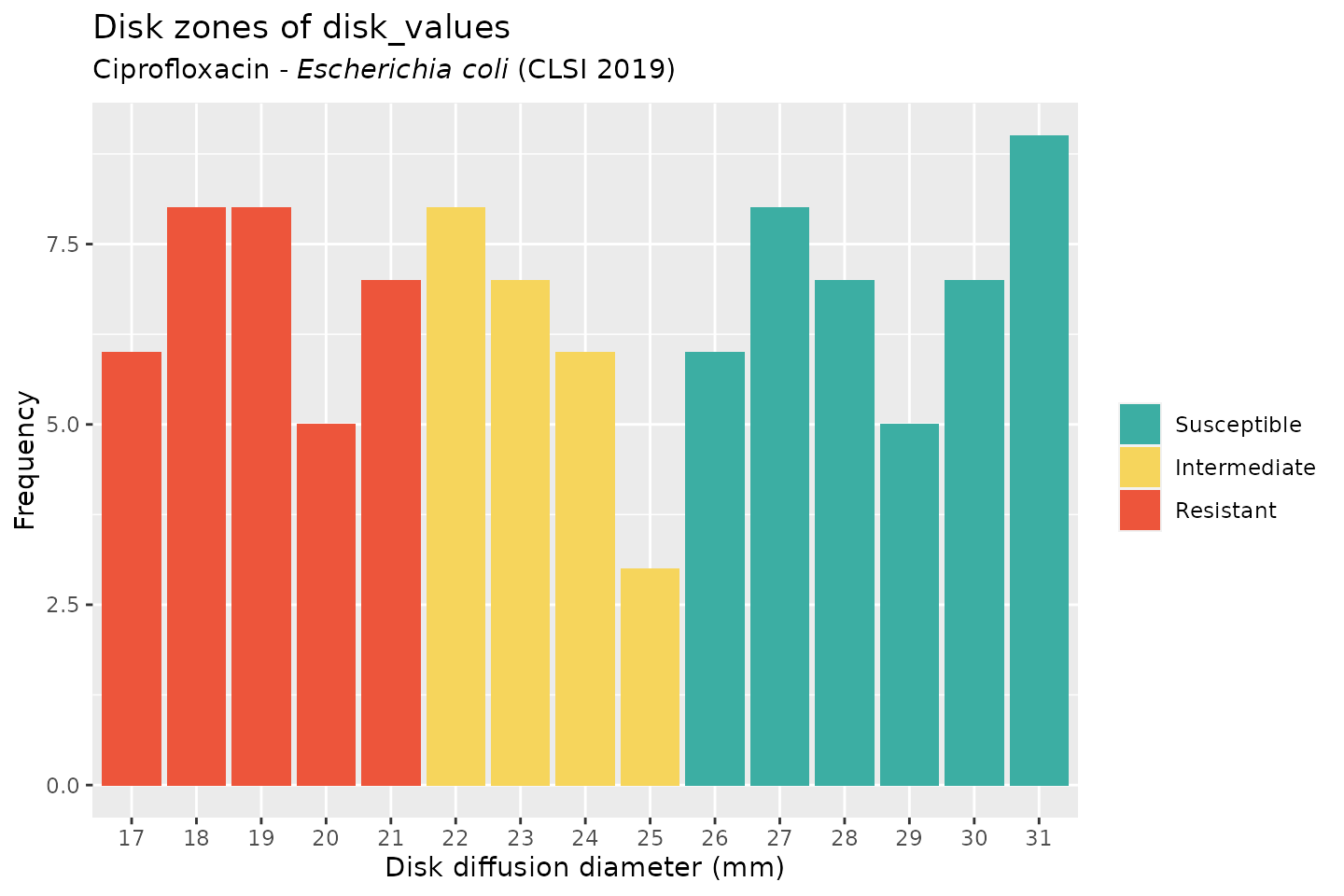
And when using the ggplot2 package, but now choosing the latest implemented CLSI guideline (notice that the EUCAST-specific term “Incr. exposure” has changed to “Intermediate”):
ggplot(disk_values, mo = "E. coli", ab = "cipro", guideline = "CLSI")
Independence test
The next example uses the example_isolates data set. This is a data set included with this package and contains 2,000 microbial isolates with their full antibiograms. It reflects reality and can be used to practice AMR data analysis.
We will compare the resistance to fosfomycin (column FOS) in hospital A and D. The input for the fisher.test() can be retrieved with a transformation like this:
# use package 'tidyr' to pivot data: library(tidyr) check_FOS <- example_isolates %>% filter(hospital_id %in% c("A", "D")) %>% # filter on only hospitals A and D select(hospital_id, FOS) %>% # select the hospitals and fosfomycin group_by(hospital_id) %>% # group on the hospitals count_df(combine_SI = TRUE) %>% # count all isolates per group (hospital_id) pivot_wider(names_from = hospital_id, # transform output so A and D are columns values_from = value) %>% select(A, D) %>% # and only select these columns as.matrix() # transform to a good old matrix for fisher.test() check_FOS # A D # [1,] 25 77 # [2,] 24 33
We can apply the test now with:
# do Fisher's Exact Test fisher.test(check_FOS) # # Fisher's Exact Test for Count Data # # data: check_FOS # p-value = 0.03104 # alternative hypothesis: true odds ratio is not equal to 1 # 95 percent confidence interval: # 0.2111489 0.9485124 # sample estimates: # odds ratio # 0.4488318
As can be seen, the p value is 0.031, which means that the fosfomycin resistance found in isolates from patients in hospital A and D are really different.