AMR
An R package to simplify the analysis and prediction of Antimicrobial Resistance (AMR) and work with antibiotic properties by using evidence-based methods.
This R package was created for academic research by PhD students of the Faculty of Medical Sciences of the University of Groningen and the Medical Microbiology & Infection Prevention (MMBI) department of the University Medical Center Groningen (UMCG).
▶️ Get it with install.packages("AMR") or see below for other possibilities. Read all changes and new functions in NEWS.md.
Authors
Matthijs S. Berends1,2,a,
Christian F. Luz1,a,
Erwin E.A. Hassing2,
Corinna Glasner1,b,
Alex W. Friedrich1,b,
Bhanu Sinha1,b
1 Department of Medical Microbiology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands - rug.nl umcg.nl
2 Certe Medical Diagnostics & Advice, Groningen, the Netherlands - certe.nl
a R package author and thesis dissertant
b Thesis advisor
Contents
Why this package?
This R package was intended to make microbial epidemiology easier. Most functions contain extensive help pages to get started.
This AMR package basically does four important things:
-
It cleanses existing data, by transforming it to reproducible and profound classes, making the most efficient use of R. These functions all use artificial intelligence to guess results that you would expect:
- Use
as.moto get an ID of a microorganism. The IDs are quite obvious - the ID of E. coli is "ESCCOL" and the ID of S. aureus is "STAAUR". The function takes almost any text as input that looks like the name or code of a microorganism like "E. coli", "esco" and "esccol". Evenas.mo("MRSA")will return the ID of S. aureus. Moreover, it can group all coagulase negative and positive Staphylococci, and can transform Streptococci into Lancefield groups. To find bacteria based on your input, this package contains a freely available database of almost 3,000 different (potential) human pathogenic microorganisms. - Use
as.rsito transform values to valid antimicrobial results. It produces just S, I or R based on your input and warns about invalid values. Even values like "<=0.002; S" (combined MIC/RSI) will result in "S". - Use
as.micto cleanse your MIC values. It produces a so-called factor (called ordinal in SPSS) with valid MIC values as levels. A value like "<=0.002; S" (combined MIC/RSI) will result in "<=0.002". - Use
as.atcto get the ATC code of an antibiotic as defined by the WHO. This package contains a database with most LIS codes, official names, DDDs and even trade names of antibiotics. For example, the values "Furabid", "Furadantin", "nitro" all return the ATC code of Nitrofurantoine.
- Use
-
It enhances existing data and adds new data from data sets included in this package.
- Use
EUCAST_rulesto apply EUCAST expert rules to isolates. - Use
first_isolateto identify the first isolates of every patient using guidelines from the CLSI (Clinical and Laboratory Standards Institute).- You can also identify first weighted isolates of every patient, an adjusted version of the CLSI guideline. This takes into account key antibiotics of every strain and compares them.
- Use
MDRO(abbreviation of Multi Drug Resistant Organisms) to check your isolates for exceptional resistance with country-specific guidelines or EUCAST rules. Currently, national guidelines for Germany and the Netherlands are supported. - The data set
microorganismscontains the family, genus, species, subspecies, colloquial name and Gram stain of almost 3,000 potential human pathogenic microorganisms (bacteria, fungi/yeasts and parasites). This enables resistance analysis of e.g. different antibiotics per Gram stain. The package also contains functions to look up values in this data set likemo_genus,mo_familyormo_gramstain. As they useas.mointernally, they also use artificial intelligence. For example,mo_genus("MRSA")andmo_genus("S. aureus")will both return"Staphylococcus". They also come with support for German, Dutch, French, Italian, Spanish and Portuguese. These functions can be used to add new variables to your data. - The data set
antibioticscontains the ATC code, LIS codes, official name, trivial name and DDD of both oral and parenteral administration. It also contains a total of 298 trade names. Use functions likeab_officialandab_tradenamesto look up values. As themo_*functions useas.mointernally, theab_*functions useas.atcinternally so it uses AI to guess your expected result. For example,ab_official("Fluclox"),ab_official("Floxapen")andab_official("J01CF05")will all return"Flucloxacillin". These functions can again be used to add new variables to your data.
- Use
-
It analyses the data with convenient functions that use well-known methods.
- Calculate the resistance (and even co-resistance) of microbial isolates with the
portion_R,portion_IR,portion_I,portion_SIandportion_Sfunctions. Similarly, the amount of isolates can be determined with thecount_R,count_IR,count_I,count_SIandcount_Sfunctions. All these functions can be used with thedplyrpackage (e.g. in conjunction withsummarise) - Plot AMR results with
geom_rsi, a function made for theggplot2package - Predict antimicrobial resistance for the nextcoming years using logistic regression models with the
resistance_predictfunction - Conduct descriptive statistics to enhance base R: calculate kurtosis, skewness and create frequency tables
- Calculate the resistance (and even co-resistance) of microbial isolates with the
-
It teaches the user how to use all the above actions.
- The package contains extensive help pages with many examples.
- It also contains an example data set called
septic_patients. This data set contains:- 2,000 blood culture isolates from anonymised septic patients between 2001 and 2017 in the Northern Netherlands
- Results of 40 antibiotics (each antibiotic in its own column) with a total of 38,414 antimicrobial results
- Real and genuine data
How to get it?
All stable versions of this package are published on CRAN, the official R network with a peer-reviewed submission process.
Install from CRAN
(Note: Downloads measured only by cran.rstudio.com, this excludes e.g. the official cran.r-project.org)
-
Install using RStudio (recommended):
- Click on
Toolsand thenInstall Packages... - Type in
AMRand press Install
- Click on
-
install.packages("AMR")
Install from GitHub
This is the latest development version. Although it may contain bugfixes and even new functions compared to the latest released version on CRAN, it is also subject to change and may be unstable or behave unexpectedly. Always consider this a beta version. All below 'badges' should be green:
| Development Test | Result | Reference |
|---|---|---|
| Works on Linux and macOS | Checked by Travis CI, GmbH [ref 1] | |
| Works on Windows | Checked by Appveyor Systems Inc. [ref 2] | |
| Syntax lines checked | Checked by Codecov LLC [ref 3] |
If so, try it with:
install.packages("devtools")
devtools::install_github("msberends/AMR")
Install from Zenodo
This package was also published on Zenodo: https://doi.org/10.5281/zenodo.1305355
How to use it?
# Call it with:
library(AMR)
# For a list of functions:
help(package = "AMR")
New classes
This package contains two new S3 classes: mic for MIC values (e.g. from Vitek or Phoenix) and rsi for antimicrobial drug interpretations (i.e. S, I and R). Both are actually ordered factors under the hood (an MIC of 2 being higher than <=1 but lower than >=32, and for class rsi factors are ordered as S < I < R).
Both classes have extensions for existing generic functions like print, summary and plot.
These functions also try to coerce valid values.
RSI
The septic_patients data set comes with antimicrobial results of more than 40 different drugs. For example, columns amox and cipr contain results of amoxicillin and ciprofloxacin, respectively.
summary(septic_patients[, c("amox", "cipr")])
# amox cipr
# Mode :rsi Mode :rsi
# <NA> :1002 <NA> :596
# Sum S :336 Sum S :1108
# Sum IR:662 Sum IR:296
# -Sum R:659 -Sum R:227
# -Sum I:3 -Sum I:69
You can use the plot function from base R:
plot(septic_patients$cipr)
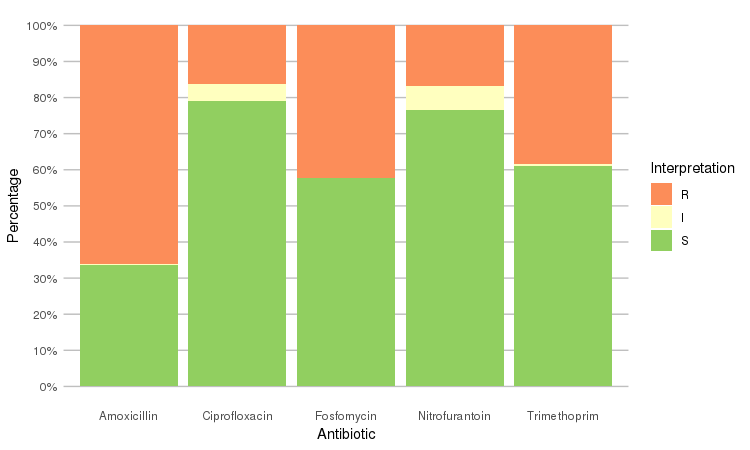
Or use the ggplot2 and dplyr packages to create more appealing plots:
library(dplyr)
library(ggplot2)
septic_patients %>%
select(amox, nitr, fosf, trim, cipr) %>%
ggplot_rsi()
Adjust it with any parameter you know from the ggplot2 package:
septic_patients %>%
select(amox, nitr, fosf, trim, cipr) %>%
ggplot_rsi(width = 0.5, colour = "black", size = 1, linetype = 2, alpha = 0.25)
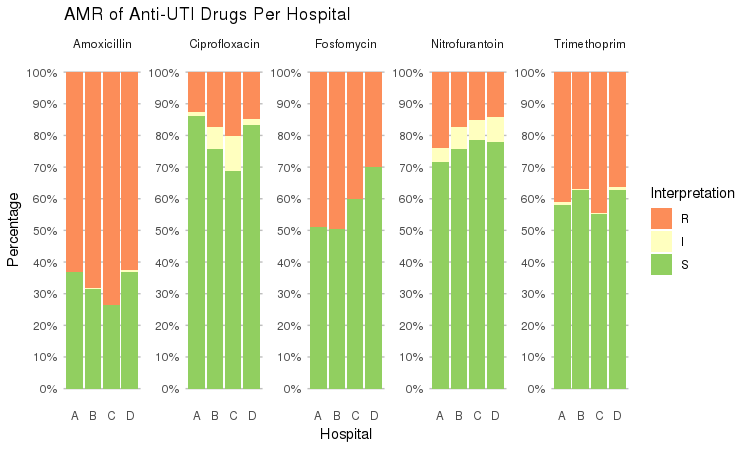
It also supports grouping variables. Let's say we want to compare resistance of drugs against Urine Tract Infections (UTI) between hospitals A to D (variable hospital_id):
septic_patients %>%
select(hospital_id, amox, nitr, fosf, trim, cipr) %>%
group_by(hospital_id) %>%
ggplot_rsi(x = "hospital_id",
facet = "Antibiotic",
nrow = 1) +
labs(title = "AMR of Anti-UTI Drugs Per Hospital",
x = "Hospital")
You could use this to group on anything in your plots: Gram stain, age (group), genus, geographic location, et cetera.
MIC
# Transform values to new class
mic_data <- as.mic(c(">=32", "1.0", "8", "<=0.128", "8", "16", "16"))
summary(mic_data)
# Mode:mic
# <NA>:0
# Min.:<=0.128
# Max.:>=32
plot(mic_data)
Overwrite/force resistance based on EUCAST rules
This is also called interpretive reading.
before <- data.frame(bact = c("STAAUR", # Staphylococcus aureus
"ENCFAE", # Enterococcus faecalis
"ESCCOL", # Escherichia coli
"KLEPNE", # Klebsiella pneumoniae
"PSEAER"), # Pseudomonas aeruginosa
vanc = "-", # Vancomycin
amox = "-", # Amoxicillin
coli = "-", # Colistin
cfta = "-", # Ceftazidime
cfur = "-", # Cefuroxime
stringsAsFactors = FALSE)
before
# bact vanc amox coli cfta cfur
# 1 STAAUR - - - - -
# 2 ENCFAE - - - - -
# 3 ESCCOL - - - - -
# 4 KLEPNE - - - - -
# 5 PSEAER - - - - -
# Now apply those rules; just need a column with bacteria IDs and antibiotic results:
after <- EUCAST_rules(before, col_mo = "bact")
after
# bact vanc amox coli cfta cfur
# 1 STAAUR - - R R -
# 2 ENCFAE - - R R R
# 3 ESCCOL R - - - -
# 4 KLEPNE R R - - -
# 5 PSEAER R R - - R
Bacteria IDs can be retrieved with the guess_mo function. It uses any type of info about a microorganism as input. For example, all these will return value STAAUR, the ID of S. aureus:
guess_mo("stau")
guess_mo("STAU")
guess_mo("staaur")
guess_mo("S. aureus")
guess_mo("S aureus")
guess_mo("Staphylococcus aureus")
guess_mo("MRSA") # Methicillin Resistant S. aureus
guess_mo("VISA") # Vancomycin Intermediate S. aureus
guess_mo("VRSA") # Vancomycin Resistant S. aureus
Other (microbial) epidemiological functions
# G-test to replace Chi squared test
g.test(...)
# Determine key antibiotic based on bacteria ID
key_antibiotics(...)
# Selection of first isolates of any patient
first_isolate(...)
# Calculate resistance levels of antibiotics, can be used with `summarise` (dplyr)
rsi(...)
# Predict resistance levels of antibiotics
rsi_predict(...)
# Get name of antibiotic by ATC code
abname(...)
abname("J01CR02", from = "atc", to = "umcg") # "AMCL"
Frequency tables
Base R lacks a simple function to create frequency tables. We created such a function that works with almost all data types: freq (or frequency_tbl). It can be used in two ways:
# Like base R:
freq(mydata$myvariable)
# And like tidyverse:
mydata %>% freq(myvariable)
Factors sort on item by default:
septic_patients %>% freq(hospital_id)
# Frequency table of `hospital_id`
# Class: factor
# Length: 2000 (of which NA: 0 = 0.0%)
# Unique: 4
#
# Item Count Percent Cum. Count Cum. Percent (Factor Level)
# --- ----- ------ -------- ----------- ------------- ---------------
# 1 A 319 16.0% 319 16.0% 1
# 2 B 661 33.1% 980 49.0% 2
# 3 C 256 12.8% 1236 61.8% 3
# 4 D 764 38.2% 2000 100.0% 4
This can be changed with the sort.count parameter:
septic_patients %>% freq(hospital_id, sort.count = TRUE)
# Frequency table of `hospital_id`
# Class: factor
# Length: 2000 (of which NA: 0 = 0.0%)
# Unique: 4
#
# Item Count Percent Cum. Count Cum. Percent (Factor Level)
# --- ----- ------ -------- ----------- ------------- ---------------
# 1 D 764 38.2% 764 38.2% 4
# 2 B 661 33.1% 1425 71.2% 2
# 3 A 319 16.0% 1744 87.2% 1
# 4 C 256 12.8% 2000 100.0% 3
All other types, like numbers, characters and dates, sort on count by default:
septic_patients %>% freq(date)
# Frequency table of `date`
# Class: Date
# Length: 2000 (of which NA: 0 = 0.0%)
# Unique: 1151
#
# Oldest: 2 January 2002
# Newest: 28 December 2017 (+5839)
# Median: 7 Augustus 2009 (~48%)
#
# Item Count Percent Cum. Count Cum. Percent
# --- ----------- ------ -------- ----------- -------------
# 1 2016-05-21 10 0.5% 10 0.5%
# 2 2004-11-15 8 0.4% 18 0.9%
# 3 2013-07-29 8 0.4% 26 1.3%
# 4 2017-06-12 8 0.4% 34 1.7%
# 5 2015-11-19 7 0.4% 41 2.1%
# 6 2005-12-22 6 0.3% 47 2.4%
# 7 2015-10-12 6 0.3% 53 2.6%
# 8 2002-05-16 5 0.2% 58 2.9%
# 9 2004-02-02 5 0.2% 63 3.1%
# 10 2004-02-18 5 0.2% 68 3.4%
# 11 2005-08-16 5 0.2% 73 3.6%
# 12 2005-09-01 5 0.2% 78 3.9%
# 13 2006-06-29 5 0.2% 83 4.2%
# 14 2007-08-10 5 0.2% 88 4.4%
# 15 2008-08-29 5 0.2% 93 4.7%
# [ reached getOption("max.print.freq") -- omitted 1136 entries, n = 1907 (95.3%) ]
For numeric values, some extra descriptive statistics will be calculated:
freq(runif(n = 10, min = 1, max = 5))
# Frequency table
# Class: numeric
# Length: 10 (of which NA: 0 = 0.0%)
# Unique: 10
#
# Mean: 3.4
# Std. dev.: 1.3 (CV: 0.38, MAD: 1.3)
# Five-Num: 1.6 | 2.0 | 3.9 | 4.7 | 4.8 (IQR: 2.7, CQV: 0.4)
# Outliers: 0
#
# Item Count Percent Cum. Count Cum. Percent
# --- --------- ------ -------- ----------- -------------
# 1 1.568997 1 10.0% 1 10.0%
# 2 1.993575 1 10.0% 2 20.0%
# 3 2.022348 1 10.0% 3 30.0%
# 4 2.236038 1 10.0% 4 40.0%
# 5 3.579828 1 10.0% 5 50.0%
# 6 4.178081 1 10.0% 6 60.0%
# 7 4.394818 1 10.0% 7 70.0%
# 8 4.689871 1 10.0% 8 80.0%
# 9 4.698626 1 10.0% 9 90.0%
# 10 4.751488 1 10.0% 10 100.0%
#
# Warning message:
# All observations are unique.
Learn more about this function with:
?freq
Data sets included in package
Datasets to work with antibiotics and bacteria properties.
# Dataset with 2000 random blood culture isolates from anonymised
# septic patients between 2001 and 2017 in 5 Dutch hospitals
septic_patients # A tibble: 2,000 x 49
# Dataset with ATC antibiotics codes, official names, trade names
# and DDDs (oral and parenteral)
antibiotics # A tibble: 423 x 18
# Dataset with bacteria codes and properties like gram stain and
# aerobic/anaerobic
microorganisms # A tibble: 2,630 x 10
Copyright
This R package is licensed under the GNU General Public License (GPL) v2.0. In a nutshell, this means that this package:
-
May be used for commercial purposes
-
May be used for private purposes
-
May not be used for patent purposes
-
May be modified, although:
- Modifications must be released under the same license when distributing the package
- Changes made to the code must be documented
-
May be distributed, although:
- Source code must be made available when the package is distributed
- A copy of the license and copyright notice must be included with the package.
-
Comes with a LIMITATION of liability
-
Comes with NO warranty