44 KiB
Conduct AMR data analysis
Note: values on this page will change with every website update since they are based on randomly created values and the page was written in R Markdown. However, the methodology remains unchanged. This page was generated on 15 December 2025.
Introduction
Conducting AMR data analysis unfortunately requires in-depth knowledge from different scientific fields, which makes it hard to do right. At least, it requires:
- Good questions (always start with those!) and reliable data
- A thorough understanding of (clinical) epidemiology, to understand the clinical and epidemiological relevance and possible bias of results
- A thorough understanding of (clinical) microbiology/infectious diseases, to understand which microorganisms are causal to which infections and the implications of pharmaceutical treatment, as well as understanding intrinsic and acquired microbial resistance
- Experience with data analysis with microbiological tests and their results, to understand the determination and limitations of MIC values and their interpretations to SIR values
- Availability of the biological taxonomy of microorganisms and probably normalisation factors for pharmaceuticals, such as defined daily doses (DDD)
- Available (inter-)national guidelines, and profound methods to apply them
Of course, we cannot instantly provide you with knowledge and
experience. But with this AMR package, we aimed at providing (1) tools
to simplify antimicrobial resistance data cleaning, transformation and
analysis, (2) methods to easily incorporate international guidelines and
(3) scientifically reliable reference data, including the requirements
mentioned above.
The AMR package enables standardised and reproducible AMR data
analysis, with the application of evidence-based rules, determination of
first isolates, translation of various codes for microorganisms and
antimicrobial agents, determination of (multi-drug) resistant
microorganisms, and calculation of antimicrobial resistance, prevalence
and future trends.
Preparation
For this tutorial, we will create fake demonstration data to work with.
You can skip to Cleaning the data if you already have your own data ready. If you start your analysis, try to make the structure of your data generally look like this:
| date | patient_id | mo | AMX | CIP |
|---|---|---|---|---|
| 2025-12-15 | abcd | Escherichia coli | S | S |
| 2025-12-15 | abcd | Escherichia coli | S | R |
| 2025-12-15 | efgh | Escherichia coli | R | S |
Needed R packages
As with many uses in R, we need some additional packages for AMR data
analysis. Our package works closely together with the tidyverse
packages
dplyr and
ggplot2 by RStudio. The tidyverse
tremendously improves the way we conduct data science - it allows for a
very natural way of writing syntaxes and creating beautiful plots in R.
We will also use the cleaner package, that can be used for cleaning
data and creating frequency tables.
library(dplyr)
library(ggplot2)
library(AMR)
# (if not yet installed, install with:)
# install.packages(c("dplyr", "ggplot2", "AMR"))
The AMR package contains a data set example_isolates_unclean, which
might look data that users have extracted from their laboratory systems:
example_isolates_unclean
#> # A tibble: 3,000 × 8
#> patient_id hospital date bacteria AMX AMC CIP GEN
#> <chr> <chr> <date> <chr> <chr> <chr> <chr> <chr>
#> 1 J3 A 2012-11-21 E. coli R I S S
#> 2 R7 A 2018-04-03 K. pneumoniae R I S S
#> 3 P3 A 2014-09-19 E. coli R S S S
#> 4 P10 A 2015-12-10 E. coli S I S S
#> 5 B7 A 2015-03-02 E. coli S S S S
#> 6 W3 A 2018-03-31 S. aureus R S R S
#> 7 J8 A 2016-06-14 E. coli R S S S
#> 8 M3 A 2015-10-25 E. coli R S S S
#> 9 J3 A 2019-06-19 E. coli S S S S
#> 10 G6 A 2015-04-27 S. aureus S S S S
#> # ℹ 2,990 more rows
# we will use 'our_data' as the data set name for this tutorial
our_data <- example_isolates_unclean
For AMR data analysis, we would like the microorganism column to contain valid, up-to-date taxonomy, and the antibiotic columns to be cleaned as SIR values as well.
Taxonomy of microorganisms
With as.mo(), users can
transform arbitrary microorganism names or codes to current taxonomy.
The AMR package contains up-to-date taxonomic data. To be specific,
currently included data were retrieved on 24 Jun 2024.
The codes of the AMR packages that come from
as.mo() are short, but
still human readable. More importantly,
as.mo() supports all kinds
of input:
as.mo("Klebsiella pneumoniae")
#> Class 'mo'
#> [1] B_KLBSL_PNMN
as.mo("K. pneumoniae")
#> Class 'mo'
#> [1] B_KLBSL_PNMN
as.mo("KLEPNE")
#> Class 'mo'
#> [1] B_KLBSL_PNMN
as.mo("KLPN")
#> Class 'mo'
#> [1] B_KLBSL_PNMN
The first character in above codes denote their taxonomic kingdom, such as Bacteria (B), Fungi (F), and Protozoa (P).
The AMR package also contain functions to directly retrieve taxonomic
properties, such as the name, genus, species, family, order, and even
Gram-stain. They all start with mo_ and they use
as.mo() internally, so
that still any arbitrary user input can be used:
mo_family("K. pneumoniae")
#> [1] "Enterobacteriaceae"
mo_genus("K. pneumoniae")
#> [1] "Klebsiella"
mo_species("K. pneumoniae")
#> [1] "pneumoniae"
mo_gramstain("Klebsiella pneumoniae")
#> [1] "Gram-negative"
mo_ref("K. pneumoniae")
#> [1] "Trevisan, 1887"
mo_snomed("K. pneumoniae")
#> [[1]]
#> [1] "1098101000112102" "446870005" "1098201000112108" "409801009"
#> [5] "56415008" "714315002" "713926009"
Now we can thus clean our data:
our_data$bacteria <- as.mo(our_data$bacteria, info = TRUE)
#> ℹ Retrieved values from the `microorganisms.codes` data set for "ESCCOL",
#> "KLEPNE", "STAAUR", and "STRPNE".
#> ℹ Microorganism translation was uncertain for four microorganisms. Run
#> `mo_uncertainties()` to review these uncertainties, or use
#> `add_custom_microorganisms()` to add custom entries.
Apparently, there was some uncertainty about the translation to taxonomic codes. Let’s check this:
mo_uncertainties()
#> Matching scores are based on the resemblance between the input and the full
#> taxonomic name, and the pathogenicity in humans. See `?mo_matching_score`.
#> Colour keys: 0.000-0.549 0.550-0.649 0.650-0.749 0.750-1.000
#>
#> --------------------------------------------------------------------------------
#> "E. coli" -> Escherichia coli (B_ESCHR_COLI, 0.688)
#> Also matched: Enterococcus crotali (0.650), Escherichia coli coli
#> (0.643), Escherichia coli expressing (0.611), Enterobacter cowanii
#> (0.600), Enterococcus columbae (0.595), Enterococcus camelliae (0.591),
#> Enterococcus casseliflavus (0.577), Enterobacter cloacae cloacae
#> (0.571), Enterobacter cloacae complex (0.571), and Enterobacter cloacae
#> dissolvens (0.565)
#> --------------------------------------------------------------------------------
#> "K. pneumoniae" -> Klebsiella pneumoniae (B_KLBSL_PNMN, 0.786)
#> Also matched: Klebsiella pneumoniae complex (0.707), Klebsiella
#> pneumoniae ozaenae (0.707), Klebsiella pneumoniae pneumoniae (0.688),
#> Klebsiella pneumoniae rhinoscleromatis (0.658), Klebsiella pasteurii
#> (0.500), Klebsiella planticola (0.500), Kingella potus (0.400),
#> Kluyveromyces pseudotropicale (0.386), Kluyveromyces pseudotropicalis
#> (0.363), and Kosakonia pseudosacchari (0.361)
#> --------------------------------------------------------------------------------
#> "S. aureus" -> Staphylococcus aureus (B_STPHY_AURS, 0.690)
#> Also matched: Staphylococcus aureus aureus (0.643), Staphylococcus
#> argenteus (0.625), Staphylococcus aureus anaerobius (0.625),
#> Staphylococcus auricularis (0.615), Salmonella Aurelianis (0.595),
#> Salmonella Aarhus (0.588), Salmonella Amounderness (0.587),
#> Staphylococcus argensis (0.587), Streptococcus australis (0.587), and
#> Salmonella choleraesuis arizonae (0.562)
#> --------------------------------------------------------------------------------
#> "S. pneumoniae" -> Streptococcus pneumoniae (B_STRPT_PNMN, 0.750)
#> Also matched: Streptococcus pseudopneumoniae (0.700), Streptococcus
#> phocae salmonis (0.552), Serratia proteamaculans quinovora (0.545),
#> Streptococcus pseudoporcinus (0.536), Staphylococcus piscifermentans
#> (0.533), Staphylococcus pseudintermedius (0.532), Serratia
#> proteamaculans proteamaculans (0.526), Streptococcus gallolyticus
#> pasteurianus (0.526), Salmonella Portanigra (0.524), and Streptococcus
#> periodonticum (0.519)
#>
#> Only the first 10 other matches of each record are shown. Run
#> `print(mo_uncertainties(), n = ...)` to view more entries, or save
#> `mo_uncertainties()` to an object.
That’s all good.
Antibiotic results
The column with antibiotic test results must also be cleaned. The AMR
package comes with three new data types to work with such test results:
mic for minimal inhibitory concentrations (MIC), disk for disk
diffusion diameters, and sir for SIR data that have been interpreted
already. This package can also determine SIR values based on MIC or disk
diffusion values, read more about that on the
as.sir() page.
For now, we will just clean the SIR columns in our data using dplyr:
# method 1, be explicit about the columns:
our_data <- our_data %>%
mutate_at(vars(AMX:GEN), as.sir)
# method 2, let the AMR package determine the eligible columns
our_data <- our_data %>%
mutate_if(is_sir_eligible, as.sir)
# result:
our_data
#> # A tibble: 3,000 × 8
#> patient_id hospital date bacteria AMX AMC CIP GEN
#> <chr> <chr> <date> <mo> <sir> <sir> <sir> <sir>
#> 1 J3 A 2012-11-21 B_ESCHR_COLI R I S S
#> 2 R7 A 2018-04-03 B_KLBSL_PNMN R I S S
#> 3 P3 A 2014-09-19 B_ESCHR_COLI R S S S
#> 4 P10 A 2015-12-10 B_ESCHR_COLI S I S S
#> 5 B7 A 2015-03-02 B_ESCHR_COLI S S S S
#> 6 W3 A 2018-03-31 B_STPHY_AURS R S R S
#> 7 J8 A 2016-06-14 B_ESCHR_COLI R S S S
#> 8 M3 A 2015-10-25 B_ESCHR_COLI R S S S
#> 9 J3 A 2019-06-19 B_ESCHR_COLI S S S S
#> 10 G6 A 2015-04-27 B_STPHY_AURS S S S S
#> # ℹ 2,990 more rows
This is basically it for the cleaning, time to start the data inclusion.
First isolates
We need to know which isolates we can actually use for analysis without repetition bias.
To conduct an analysis of antimicrobial resistance, you must only include the first isolate of every patient per episode (Hindler et al., Clin Infect Dis. 2007). If you would not do this, you could easily get an overestimate or underestimate of the resistance of an antibiotic. Imagine that a patient was admitted with an MRSA and that it was found in 5 different blood cultures the following weeks (yes, some countries like the Netherlands have these blood drawing policies). The resistance percentage of oxacillin of all isolates would be overestimated, because you included this MRSA more than once. It would clearly be selection bias.
The Clinical and Laboratory Standards Institute (CLSI) appoints this as follows:
(…) When preparing a cumulative antibiogram to guide clinical decisions about empirical antimicrobial therapy of initial infections, only the first isolate of a given species per patient, per analysis period (eg, one year) should be included, irrespective of body site, antimicrobial susceptibility profile, or other phenotypical characteristics (eg, biotype). The first isolate is easily identified, and cumulative antimicrobial susceptibility test data prepared using the first isolate are generally comparable to cumulative antimicrobial susceptibility test data calculated by other methods, providing duplicate isolates are excluded.
M39-A4 Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data, 4th Edition. CLSI, 2014. Chapter 6.4
This AMR package includes this methodology with the
first_isolate()
function and is able to apply the four different methods as defined by
Hindler et al. in
2007:
phenotype-based, episode-based, patient-based, isolate-based. The right
method depends on your goals and analysis, but the default
phenotype-based method is in any case the method to properly correct for
most duplicate isolates. Read more about the methods on the
first_isolate()
page.
The outcome of the function can easily be added to our data:
our_data <- our_data %>%
mutate(first = first_isolate(info = TRUE))
#> ℹ Determining first isolates using an episode length of 365 days
#> ℹ Using column 'bacteria' as input for `col_mo`.
#> ℹ Using column 'date' as input for `col_date`.
#> ℹ Using column 'patient_id' as input for `col_patient_id`.
#> ℹ Basing inclusion on all antimicrobial results, using a points threshold
#> of 2
#> => Found 2,730 'phenotype-based' first isolates (91.0% of total where a
#> microbial ID was available)
So only 91% is suitable for resistance analysis! We can now filter on it
with the filter()
function, also from the dplyr package:
our_data_1st <- our_data %>%
filter(first == TRUE)
For future use, the above two syntaxes can be shortened:
our_data_1st <- our_data %>%
filter_first_isolate()
So we end up with 2 730 isolates for analysis. Now our data looks like:
our_data_1st
#> # A tibble: 2,730 × 9
#> patient_id hospital date bacteria AMX AMC CIP GEN first
#> <chr> <chr> <date> <mo> <sir> <sir> <sir> <sir> <lgl>
#> 1 J3 A 2012-11-21 B_ESCHR_COLI R I S S TRUE
#> 2 R7 A 2018-04-03 B_KLBSL_PNMN R I S S TRUE
#> 3 P3 A 2014-09-19 B_ESCHR_COLI R S S S TRUE
#> 4 P10 A 2015-12-10 B_ESCHR_COLI S I S S TRUE
#> 5 B7 A 2015-03-02 B_ESCHR_COLI S S S S TRUE
#> 6 W3 A 2018-03-31 B_STPHY_AURS R S R S TRUE
#> 7 M3 A 2015-10-25 B_ESCHR_COLI R S S S TRUE
#> 8 J3 A 2019-06-19 B_ESCHR_COLI S S S S TRUE
#> 9 G6 A 2015-04-27 B_STPHY_AURS S S S S TRUE
#> 10 P4 A 2011-06-21 B_ESCHR_COLI S S S S TRUE
#> # ℹ 2,720 more rows
Time for the analysis.
Analysing the data
The base R summary() function
gives a good first impression, as it comes with support for the new mo
and sir classes that we now have in our data set:
summary(our_data_1st)
#> patient_id hospital date
#> Length:2730 Length:2730 Min. :2011-01-01
#> Class :character Class :character 1st Qu.:2013-04-06
#> Mode :character Mode :character Median :2015-06-04
#> Mean :2015-06-09
#> 3rd Qu.:2017-08-14
#> Max. :2019-12-27
#> bacteria AMX AMC
#> Class :mo Class:sir Class:sir
#> <NA> :0 %S :40.1% (n=1071) %S :51.1% (n=1354)
#> Unique:4 %SDD : 0.0% (n=0) %SDD : 0.0% (n=0)
#> #1 :B_ESCHR_COLI %I :17.0% (n=453) %I :12.7% (n=335)
#> #2 :B_STPHY_AURS %R :42.9% (n=1147) %R :36.2% (n=959)
#> #3 :B_STRPT_PNMN %NI : 0.0% (n=0) %NI : 0.0% (n=0)
#> CIP GEN first
#> Class:sir Class:sir Mode:logical
#> %S :52.2% (n=1426) %S :60.7% (n=1656) TRUE:2730
#> %SDD : 0.0% (n=0) %SDD : 0.0% (n=0)
#> %I : 6.5% (n=178) %I : 3.0% (n=83)
#> %R :41.2% (n=1126) %R :36.3% (n=991)
#> %NI : 0.0% (n=0) %NI : 0.0% (n=0)
glimpse(our_data_1st)
#> Rows: 2,730
#> Columns: 9
#> $ patient_id <chr> "J3", "R7", "P3", "P10", "B7", "W3", "M3", "J3", "G6", "P4"…
#> $ hospital <chr> "A", "A", "A", "A", "A", "A", "A", "A", "A", "A", "A", "A",…
#> $ date <date> 2012-11-21, 2018-04-03, 2014-09-19, 2015-12-10, 2015-03-02…
#> $ bacteria <mo> "B_ESCHR_COLI", "B_KLBSL_PNMN", "B_ESCHR_COLI", "B_ESCHR_COL…
#> $ AMX <sir> R, R, R, S, S, R, R, S, S, S, S, R, S, S, R, R, R, R, S, R,…
#> $ AMC <sir> I, I, S, I, S, S, S, S, S, S, S, S, S, S, S, S, S, R, S, S,…
#> $ CIP <sir> S, S, S, S, S, R, S, S, S, S, S, S, S, S, S, S, S, S, S, S,…
#> $ GEN <sir> S, S, S, S, S, S, S, S, S, S, S, R, S, S, S, S, S, S, S, S,…
#> $ first <lgl> TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE,…
# number of unique values per column:
sapply(our_data_1st, n_distinct)
#> patient_id hospital date bacteria AMX AMC CIP
#> 260 3 1854 4 4 4 3
#> GEN first
#> 3 1
Availability of species
To just get an idea how the species are distributed, create a frequency
table with count() based
on the name of the microorganisms:
our_data %>%
count(mo_name(bacteria), sort = TRUE)
#> # A tibble: 4 × 2
#> `mo_name(bacteria)` n
#> <chr> <int>
#> 1 Escherichia coli 1518
#> 2 Staphylococcus aureus 730
#> 3 Streptococcus pneumoniae 426
#> 4 Klebsiella pneumoniae 326
our_data_1st %>%
count(mo_name(bacteria), sort = TRUE)
#> # A tibble: 4 × 2
#> `mo_name(bacteria)` n
#> <chr> <int>
#> 1 Escherichia coli 1326
#> 2 Staphylococcus aureus 684
#> 3 Streptococcus pneumoniae 401
#> 4 Klebsiella pneumoniae 319
Select and filter with antibiotic selectors
Using so-called antibiotic class selectors, you can select or filter columns based on the antibiotic class that your antibiotic results are in:
our_data_1st %>%
select(date, aminoglycosides())
#> ℹ For `aminoglycosides()` using column 'GEN' (gentamicin)
#> # A tibble: 2,730 × 2
#> date GEN
#> <date> <sir>
#> 1 2012-11-21 S
#> 2 2018-04-03 S
#> 3 2014-09-19 S
#> 4 2015-12-10 S
#> 5 2015-03-02 S
#> 6 2018-03-31 S
#> 7 2015-10-25 S
#> 8 2019-06-19 S
#> 9 2015-04-27 S
#> 10 2011-06-21 S
#> # ℹ 2,720 more rows
our_data_1st %>%
select(bacteria, betalactams())
#> ℹ For `betalactams()` using columns 'AMX' (amoxicillin) and 'AMC'
#> (amoxicillin/clavulanic acid)
#> # A tibble: 2,730 × 3
#> bacteria AMX AMC
#> <mo> <sir> <sir>
#> 1 B_ESCHR_COLI R I
#> 2 B_KLBSL_PNMN R I
#> 3 B_ESCHR_COLI R S
#> 4 B_ESCHR_COLI S I
#> 5 B_ESCHR_COLI S S
#> 6 B_STPHY_AURS R S
#> 7 B_ESCHR_COLI R S
#> 8 B_ESCHR_COLI S S
#> 9 B_STPHY_AURS S S
#> 10 B_ESCHR_COLI S S
#> # ℹ 2,720 more rows
our_data_1st %>%
select(bacteria, where(is.sir))
#> # A tibble: 2,730 × 5
#> bacteria AMX AMC CIP GEN
#> <mo> <sir> <sir> <sir> <sir>
#> 1 B_ESCHR_COLI R I S S
#> 2 B_KLBSL_PNMN R I S S
#> 3 B_ESCHR_COLI R S S S
#> 4 B_ESCHR_COLI S I S S
#> 5 B_ESCHR_COLI S S S S
#> 6 B_STPHY_AURS R S R S
#> 7 B_ESCHR_COLI R S S S
#> 8 B_ESCHR_COLI S S S S
#> 9 B_STPHY_AURS S S S S
#> 10 B_ESCHR_COLI S S S S
#> # ℹ 2,720 more rows
# filtering using AB selectors is also possible:
our_data_1st %>%
filter(any(aminoglycosides() == "R"))
#> ℹ For `aminoglycosides()` using column 'GEN' (gentamicin)
#> # A tibble: 991 × 9
#> patient_id hospital date bacteria AMX AMC CIP GEN first
#> <chr> <chr> <date> <mo> <sir> <sir> <sir> <sir> <lgl>
#> 1 J5 A 2017-12-25 B_STRPT_PNMN R S S R TRUE
#> 2 X1 A 2017-07-04 B_STPHY_AURS R S S R TRUE
#> 3 B3 A 2016-07-24 B_ESCHR_COLI S S S R TRUE
#> 4 V7 A 2012-04-03 B_ESCHR_COLI S S S R TRUE
#> 5 C9 A 2017-03-23 B_ESCHR_COLI S S S R TRUE
#> 6 R1 A 2018-06-10 B_STPHY_AURS S S S R TRUE
#> 7 S2 A 2013-07-19 B_STRPT_PNMN S S S R TRUE
#> 8 P5 A 2019-03-09 B_STPHY_AURS S S S R TRUE
#> 9 Q8 A 2019-08-10 B_STPHY_AURS S S S R TRUE
#> 10 K5 A 2013-03-15 B_STRPT_PNMN S S S R TRUE
#> # ℹ 981 more rows
our_data_1st %>%
filter(all(betalactams() == "R"))
#> ℹ For `betalactams()` using columns 'AMX' (amoxicillin) and 'AMC'
#> (amoxicillin/clavulanic acid)
#> # A tibble: 461 × 9
#> patient_id hospital date bacteria AMX AMC CIP GEN first
#> <chr> <chr> <date> <mo> <sir> <sir> <sir> <sir> <lgl>
#> 1 M7 A 2013-07-22 B_STRPT_PNMN R R S S TRUE
#> 2 R10 A 2013-12-20 B_STPHY_AURS R R S S TRUE
#> 3 R7 A 2015-10-25 B_STPHY_AURS R R S S TRUE
#> 4 R8 A 2019-10-25 B_STPHY_AURS R R S S TRUE
#> 5 B6 A 2016-11-20 B_ESCHR_COLI R R R R TRUE
#> 6 I7 A 2015-08-19 B_ESCHR_COLI R R S S TRUE
#> 7 N3 A 2014-12-29 B_STRPT_PNMN R R R S TRUE
#> 8 Q2 A 2019-09-22 B_ESCHR_COLI R R S S TRUE
#> 9 X7 A 2011-03-20 B_ESCHR_COLI R R S R TRUE
#> 10 V1 A 2018-08-07 B_STPHY_AURS R R S S TRUE
#> # ℹ 451 more rows
# even works in base R (since R 3.0):
our_data_1st[all(betalactams() == "R"), ]
#> ℹ For `betalactams()` using columns 'AMX' (amoxicillin) and 'AMC'
#> (amoxicillin/clavulanic acid)
#> # A tibble: 461 × 9
#> patient_id hospital date bacteria AMX AMC CIP GEN first
#> <chr> <chr> <date> <mo> <sir> <sir> <sir> <sir> <lgl>
#> 1 M7 A 2013-07-22 B_STRPT_PNMN R R S S TRUE
#> 2 R10 A 2013-12-20 B_STPHY_AURS R R S S TRUE
#> 3 R7 A 2015-10-25 B_STPHY_AURS R R S S TRUE
#> 4 R8 A 2019-10-25 B_STPHY_AURS R R S S TRUE
#> 5 B6 A 2016-11-20 B_ESCHR_COLI R R R R TRUE
#> 6 I7 A 2015-08-19 B_ESCHR_COLI R R S S TRUE
#> 7 N3 A 2014-12-29 B_STRPT_PNMN R R R S TRUE
#> 8 Q2 A 2019-09-22 B_ESCHR_COLI R R S S TRUE
#> 9 X7 A 2011-03-20 B_ESCHR_COLI R R S R TRUE
#> 10 V1 A 2018-08-07 B_STPHY_AURS R R S S TRUE
#> # ℹ 451 more rows
Generate antibiograms
Since AMR v2.0 (March 2023), it is very easy to create different types of antibiograms, with support for 20 different languages.
There are four antibiogram types, as proposed by Klinker et al. (2021,
DOI
10.1177/20499361211011373),
and they are all supported by the new
antibiogram()
function:
- Traditional Antibiogram (TA) e.g, for the susceptibility of Pseudomonas aeruginosa to piperacillin/tazobactam (TZP)
- Combination Antibiogram (CA) e.g, for the sdditional susceptibility of Pseudomonas aeruginosa to TZP + tobramycin versus TZP alone
- Syndromic Antibiogram (SA) e.g, for the susceptibility of Pseudomonas aeruginosa to TZP among respiratory specimens (obtained among ICU patients only)
- Weighted-Incidence Syndromic Combination Antibiogram (WISCA) e.g, for the susceptibility of Pseudomonas aeruginosa to TZP among respiratory specimens (obtained among ICU patients only) for male patients age >=65 years with heart failure
In this section, we show how to use the
antibiogram()
function to create any of the above antibiogram types. For starters,
this is what the included example_isolates data set looks like:
example_isolates
#> # A tibble: 2,000 × 46
#> date patient age gender ward mo PEN OXA FLC AMX
#> <date> <chr> <dbl> <chr> <chr> <mo> <sir> <sir> <sir> <sir>
#> 1 2002-01-02 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 2 2002-01-03 A77334 65 F Clinical B_ESCHR_COLI R NA NA NA
#> 3 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 4 2002-01-07 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 5 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 6 2002-01-13 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 7 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 8 2002-01-14 462729 78 M Clinical B_STPHY_AURS R NA S R
#> 9 2002-01-16 067927 45 F ICU B_STPHY_EPDR R NA R NA
#> 10 2002-01-17 858515 79 F ICU B_STPHY_EPDR R NA S NA
#> # ℹ 1,990 more rows
#> # ℹ 36 more variables: AMC <sir>, AMP <sir>, TZP <sir>, CZO <sir>, FEP <sir>,
#> # CXM <sir>, FOX <sir>, CTX <sir>, CAZ <sir>, CRO <sir>, GEN <sir>,
#> # TOB <sir>, AMK <sir>, KAN <sir>, TMP <sir>, SXT <sir>, NIT <sir>,
#> # FOS <sir>, LNZ <sir>, CIP <sir>, MFX <sir>, VAN <sir>, TEC <sir>,
#> # TCY <sir>, TGC <sir>, DOX <sir>, ERY <sir>, CLI <sir>, AZM <sir>,
#> # IPM <sir>, MEM <sir>, MTR <sir>, CHL <sir>, COL <sir>, MUP <sir>, …
Traditional Antibiogram
To create a traditional antibiogram, simply state which antibiotics
should be used. The antibiotics argument in the
antibiogram()
function supports any (combination) of the previously mentioned
antibiotic class selectors:
antibiogram(example_isolates,
antibiotics = c(aminoglycosides(), carbapenems()))
#> ℹ For `aminoglycosides()` using columns 'GEN' (gentamicin), 'TOB'
#> (tobramycin), 'AMK' (amikacin), and 'KAN' (kanamycin)
#> ℹ For `carbapenems()` using columns 'IPM' (imipenem) and 'MEM' (meropenem)
| Pathogen | Amikacin | Gentamicin | Imipenem | Kanamycin | Meropenem | Tobramycin |
|---|---|---|---|---|---|---|
| CoNS | 0% (0-8%,N=43) | 86% (82-90%,N=309) | 52% (37-67%,N=48) | 0% (0-8%,N=43) | 52% (37-67%,N=48) | 22% (12-35%,N=55) |
| E. coli | 100% (98-100%,N=171) | 98% (96-99%,N=460) | 100% (99-100%,N=422) | NA | 100% (99-100%,N=418) | 97% (96-99%,N=462) |
| E. faecalis | 0% (0-9%,N=39) | 0% (0-9%,N=39) | 100% (91-100%,N=38) | 0% (0-9%,N=39) | NA | 0% (0-9%,N=39) |
| K. pneumoniae | NA | 90% (79-96%,N=58) | 100% (93-100%,N=51) | NA | 100% (93-100%,N=53) | 90% (79-96%,N=58) |
| P. aeruginosa | NA | 100% (88-100%,N=30) | NA | 0% (0-12%,N=30) | NA | 100% (88-100%,N=30) |
| P. mirabilis | NA | 94% (80-99%,N=34) | 94% (79-99%,N=32) | NA | NA | 94% (80-99%,N=34) |
| S. aureus | NA | 99% (97-100%,N=233) | NA | NA | NA | 98% (92-100%,N=86) |
| S. epidermidis | 0% (0-8%,N=44) | 79% (71-85%,N=163) | NA | 0% (0-8%,N=44) | NA | 51% (40-61%,N=89) |
| S. hominis | NA | 92% (84-97%,N=80) | NA | NA | NA | 85% (74-93%,N=62) |
| S. pneumoniae | 0% (0-3%,N=117) | 0% (0-3%,N=117) | NA | 0% (0-3%,N=117) | NA | 0% (0-3%,N=117) |
Notice that the
antibiogram()
function automatically prints in the right format when using Quarto or R
Markdown (such as this page), and even applies italics for taxonomic
names (by using
italicise_taxonomy()
internally).
It also uses the language of your OS if this is either English, Arabic,
Bengali, Chinese, Czech, Danish, Dutch, Finnish, French, German, Greek,
Hindi, Indonesian, Italian, Japanese, Korean, Norwegian, Polish,
Portuguese, Romanian, Russian, Spanish, Swahili, Swedish, Turkish,
Ukrainian, Urdu, or Vietnamese. In this next example, we force the
language to be Spanish using the language argument:
antibiogram(example_isolates,
mo_transform = "gramstain",
antibiotics = aminoglycosides(),
ab_transform = "name",
language = "es")
#> ℹ For `aminoglycosides()` using columns 'GEN' (gentamicin), 'TOB'
#> (tobramycin), 'AMK' (amikacin), and 'KAN' (kanamycin)
| Patógeno | Amikacina | Gentamicina | Kanamicina | Tobramicina |
|---|---|---|---|---|
| Gram negativo | 98% (96-99%,N=256) | 96% (95-98%,N=684) | 0% (0-10%,N=35) | 96% (94-97%,N=686) |
| Gram positivo | 0% (0-1%,N=436) | 63% (60-66%,N=1170) | 0% (0-1%,N=436) | 34% (31-38%,N=665) |
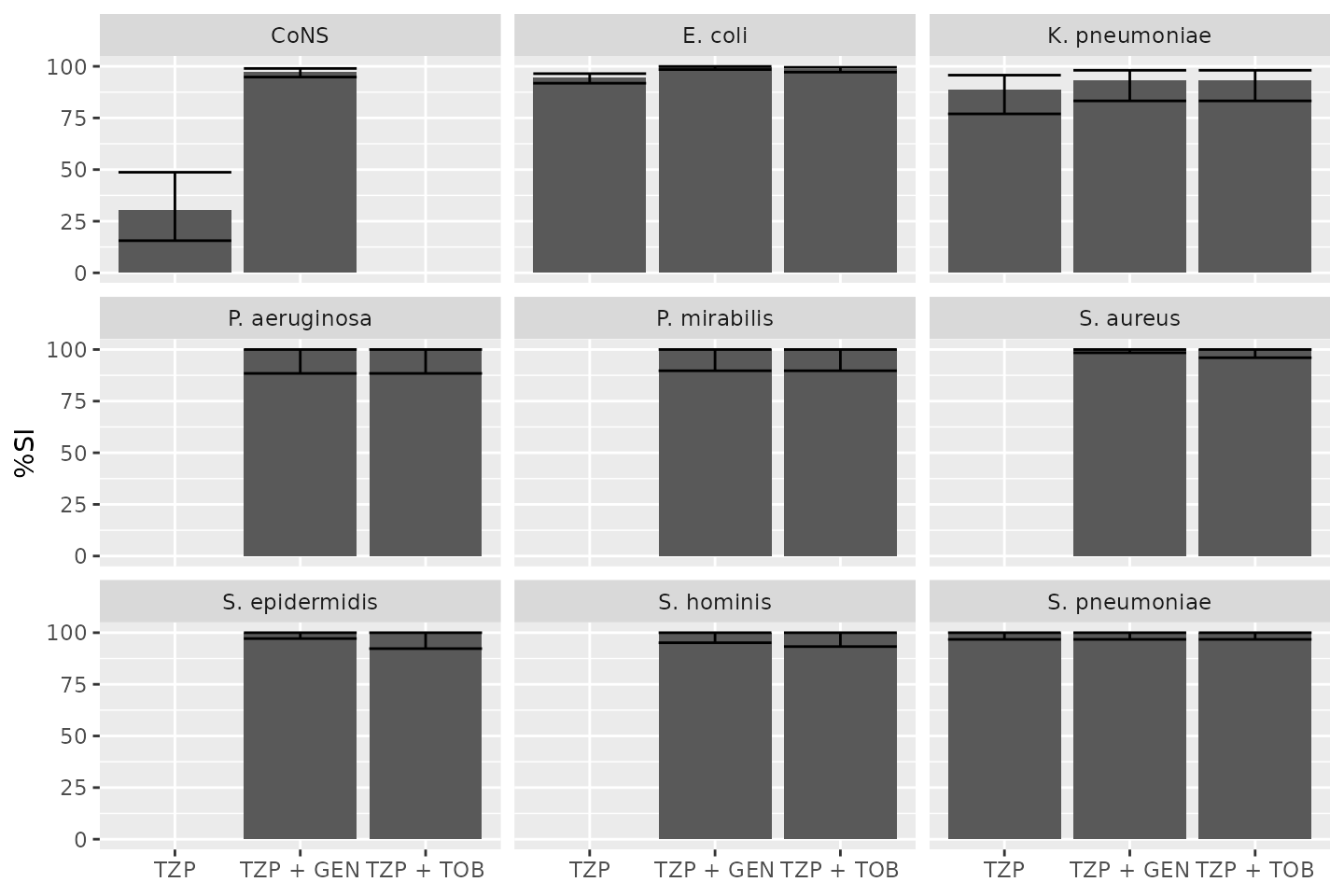
Combined Antibiogram
To create a combined antibiogram, use antibiotic codes or names with a
plus + character like this:
combined_ab <- antibiogram(example_isolates,
antibiotics = c("TZP", "TZP+TOB", "TZP+GEN"),
ab_transform = NULL)
combined_ab
| Pathogen | TZP | TZP + GEN | TZP + TOB |
|---|---|---|---|
| CoNS | 30% (16-49%,N=33) | 97% (95-99%,N=274) | NA |
| E. coli | 94% (92-96%,N=416) | 100% (98-100%,N=459) | 99% (97-100%,N=461) |
| K. pneumoniae | 89% (77-96%,N=53) | 93% (83-98%,N=58) | 93% (83-98%,N=58) |
| P. aeruginosa | NA | 100% (88-100%,N=30) | 100% (88-100%,N=30) |
| P. mirabilis | NA | 100% (90-100%,N=34) | 100% (90-100%,N=34) |
| S. aureus | NA | 100% (98-100%,N=231) | 100% (96-100%,N=91) |
| S. epidermidis | NA | 100% (97-100%,N=128) | 100% (92-100%,N=46) |
| S. hominis | NA | 100% (95-100%,N=74) | 100% (93-100%,N=53) |
| S. pneumoniae | 100% (97-100%,N=112) | 100% (97-100%,N=112) | 100% (97-100%,N=112) |
Syndromic Antibiogram
To create a syndromic antibiogram, the syndromic_group argument must
be used. This can be any column in the data, or e.g. an
ifelse() with calculations based
on certain columns:
antibiogram(example_isolates,
antibiotics = c(aminoglycosides(), carbapenems()),
syndromic_group = "ward")
#> ℹ For `aminoglycosides()` using columns 'GEN' (gentamicin), 'TOB'
#> (tobramycin), 'AMK' (amikacin), and 'KAN' (kanamycin)
#> ℹ For `carbapenems()` using columns 'IPM' (imipenem) and 'MEM' (meropenem)
| Syndromic Group | Pathogen | Amikacin | Gentamicin | Imipenem | Kanamycin | Meropenem | Tobramycin |
|---|---|---|---|---|---|---|---|
| Clinical | CoNS | NA | 89% (84-93%,N=205) | 57% (39-74%,N=35) | NA | 57% (39-74%,N=35) | 26% (12-45%,N=31) |
| ICU | CoNS | NA | 79% (68-88%,N=73) | NA | NA | NA | NA |
| Outpatient | CoNS | NA | 84% (66-95%,N=31) | NA | NA | NA | NA |
| Clinical | E. coli | 100% (97-100%,N=104) | 98% (96-99%,N=297) | 100% (99-100%,N=266) | NA | 100% (99-100%,N=276) | 98% (96-99%,N=299) |
| ICU | E. coli | 100% (93-100%,N=52) | 99% (95-100%,N=137) | 100% (97-100%,N=133) | NA | 100% (97-100%,N=118) | 96% (92-99%,N=137) |
| Clinical | K. pneumoniae | NA | 92% (81-98%,N=51) | 100% (92-100%,N=44) | NA | 100% (92-100%,N=46) | 92% (81-98%,N=51) |
| Clinical | P. mirabilis | NA | 100% (88-100%,N=30) | NA | NA | NA | 100% (88-100%,N=30) |
| Clinical | S. aureus | NA | 99% (95-100%,N=150) | NA | NA | NA | 97% (89-100%,N=63) |
| ICU | S. aureus | NA | 100% (95-100%,N=66) | NA | NA | NA | NA |
| Clinical | S. epidermidis | NA | 82% (72-90%,N=79) | NA | NA | NA | 55% (39-70%,N=44) |
| ICU | S. epidermidis | NA | 72% (60-82%,N=75) | NA | NA | NA | 41% (26-58%,N=41) |
| Clinical | S. hominis | NA | 96% (85-99%,N=45) | NA | NA | NA | 94% (79-99%,N=31) |
| Clinical | S. pneumoniae | 0% (0-5%,N=78) | 0% (0-5%,N=78) | NA | 0% (0-5%,N=78) | NA | 0% (0-5%,N=78) |
| ICU | S. pneumoniae | 0% (0-12%,N=30) | 0% (0-12%,N=30) | NA | 0% (0-12%,N=30) | NA | 0% (0-12%,N=30) |
Weighted-Incidence Syndromic Combination Antibiogram (WISCA)
To create a Weighted-Incidence Syndromic Combination Antibiogram
(WISCA), simply set wisca = TRUE in the
antibiogram()
function, or use the dedicated
wisca() function.
Unlike traditional antibiograms, WISCA provides syndrome-based
susceptibility estimates, weighted by pathogen incidence and
antimicrobial susceptibility patterns.
example_isolates %>%
wisca(antibiotics = c("TZP", "TZP+TOB", "TZP+GEN"),
minimum = 10) # Recommended threshold: ≥30
| Piperacillin/tazobactam | Piperacillin/tazobactam + Gentamicin | Piperacillin/tazobactam + Tobramycin |
|---|---|---|
| 69.4% (64.3-74.3%) | 92.6% (91.1-93.9%) | 88.7% (85.8-91.2%) |
WISCA uses a Bayesian decision model to integrate data from multiple pathogens, improving empirical therapy guidance, especially for low-incidence infections. It is pathogen-agnostic, meaning results are syndrome-based rather than stratified by microorganism.
For reliable results, ensure your data includes only first isolates
(use
first_isolate())
and consider filtering for the top n species (use
top_n_microorganisms()),
as WISCA outcomes are most meaningful when based on robust incidence
estimates.
For patient- or syndrome-specific WISCA, run the function on a
grouped tibble, i.e., using
group_by()
first:
example_isolates %>%
top_n_microorganisms(n = 10) %>%
group_by(age_group = age_groups(age, c(25, 50, 75)),
gender) %>%
wisca(antibiotics = c("TZP", "TZP+TOB", "TZP+GEN"))
| age_group | gender | Piperacillin/tazobactam | Piperacillin/tazobactam + Gentamicin | Piperacillin/tazobactam + Tobramycin |
|---|---|---|---|---|
| 0-24 | F | 56.6% (25.2-83.9%) | 73.6% (48-91.6%) | 68.6% (42.9-89.5%) |
| 0-24 | M | 60.3% (28.4-87.1%) | 79.7% (57.6-94.2%) | 60.1% (29.5-87.7%) |
| 25-49 | F | 66.6% (45.6-85.5%) | 91.7% (84.6-96.7%) | 83% (67.9-94%) |
| 25-49 | M | 56.4% (29.1-81.7%) | 89.2% (80.3-95.7%) | 72.4% (49.7-90%) |
| 50-74 | F | 67.8% (55.8-80.1%) | 95.6% (93.2-97.5%) | 88.1% (80.4-94.6%) |
| 50-74 | M | 66.2% (54.8-75.8%) | 95.2% (92.4-97.4%) | 84.4% (74.4-92.5%) |
| 75+ | F | 71.7% (61-81.7%) | 96.6% (94.4-98.2%) | 90.6% (84.6-95.3%) |
| 75+ | M | 72.9% (63.8-82%) | 96.6% (94.6-98.1%) | 92.8% (87.8-96.5%) |
Plotting antibiograms
Antibiograms can be plotted using
autoplot()
from the ggplot2 packages, since this AMR package provides an
extension to that function:
autoplot(combined_ab)
To calculate antimicrobial resistance in a more sensible way, also by
correcting for too few results, we use the
resistance() and
susceptibility()
functions.
Resistance percentages
The functions
resistance() and
susceptibility() can
be used to calculate antimicrobial resistance or susceptibility. For
more specific analyses, the functions
proportion_S(),
proportion_SI(),
proportion_I(),
proportion_IR() and
proportion_R() can be
used to determine the proportion of a specific antimicrobial outcome.
All these functions contain a minimum argument, denoting the minimum
required number of test results for returning a value. These functions
will otherwise return NA. The default is minimum = 30, following the
CLSI M39-A4
guideline
for applying microbial epidemiology.
As per the EUCAST guideline of 2019, we calculate resistance as the
proportion of R
(proportion_R(),
equal to
resistance()) and
susceptibility as the proportion of S and I
(proportion_SI(),
equal to
susceptibility()).
These functions can be used on their own:
our_data_1st %>% resistance(AMX)
#> [1] 0.4294272
Or can be used in conjunction with
group_by() and
summarise(),
both from the dplyr package:
our_data_1st %>%
group_by(hospital) %>%
summarise(amoxicillin = resistance(AMX))
#> # A tibble: 3 × 2
#> hospital amoxicillin
#> <chr> <dbl>
#> 1 A 0.341
#> 2 B 0.586
#> 3 C 0.370
Interpreting MIC and Disk Diffusion Values
Minimal inhibitory concentration (MIC) values and disk diffusion
diameters can be interpreted into clinical breakpoints (SIR) using
as.sir(). Here’s an
example with randomly generated MIC values for Klebsiella pneumoniae
and ciprofloxacin:
set.seed(123)
mic_values <- random_mic(100)
sir_values <- as.sir(mic_values, mo = "K. pneumoniae", ab = "cipro", guideline = "EUCAST 2024")
my_data <- tibble(MIC = mic_values, SIR = sir_values)
my_data
#> # A tibble: 100 × 2
#> MIC SIR
#> <mic> <sir>
#> 1 <=0.0001 S
#> 2 0.0160 S
#> 3 >=8.0000 R
#> 4 0.0320 S
#> 5 0.0080 S
#> 6 64.0000 R
#> 7 0.0080 S
#> 8 0.1250 S
#> 9 0.0320 S
#> 10 0.0002 S
#> # ℹ 90 more rows
This allows direct interpretation according to EUCAST or CLSI breakpoints, facilitating automated AMR data processing.
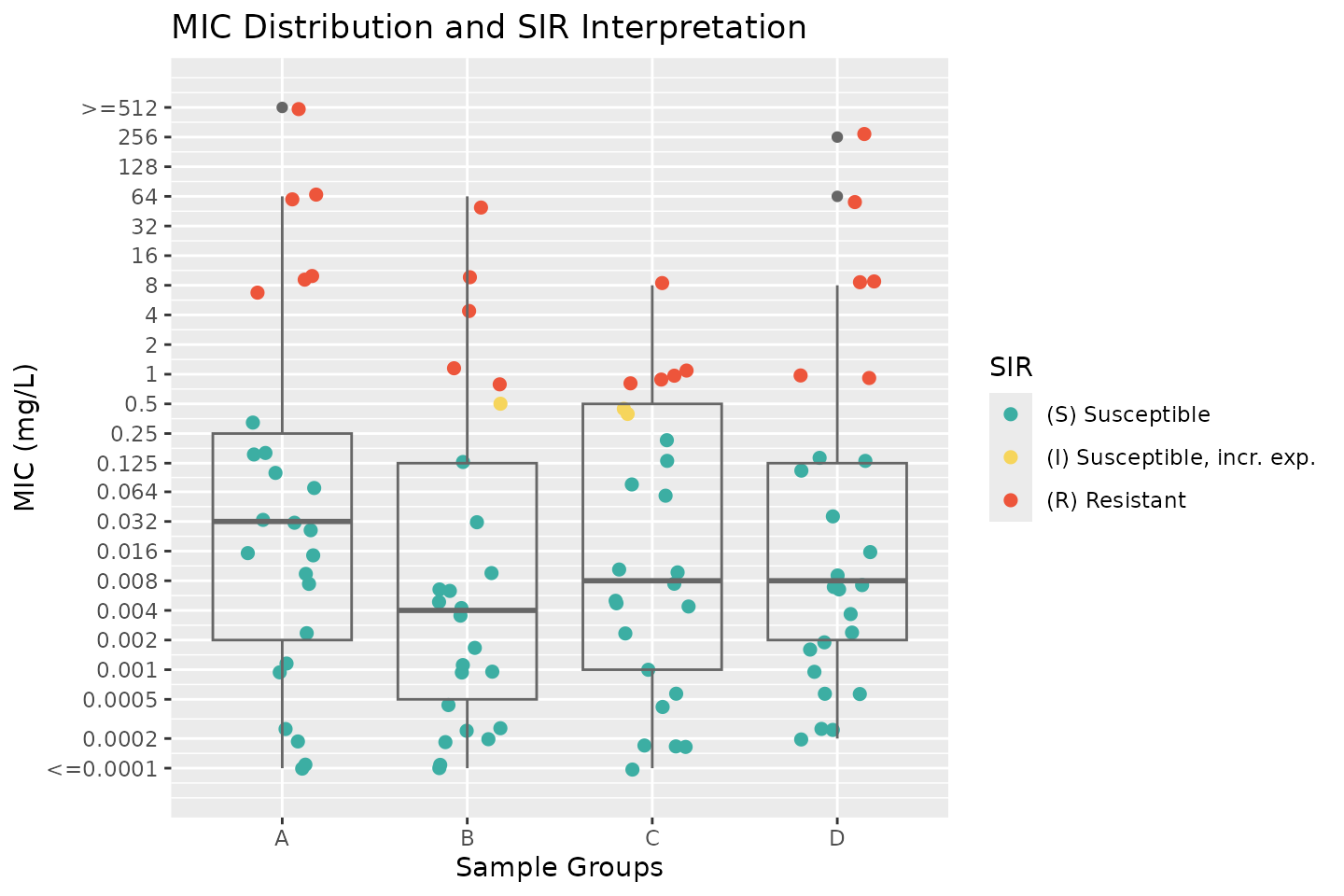
Plotting MIC and SIR Interpretations
We can visualise MIC distributions and their SIR interpretations using
ggplot2, using the new
scale_y_mic() for the
y-axis and
scale_colour_sir() to
colour-code SIR categories.
# add a group
my_data$group <- rep(c("A", "B", "C", "D"), each = 25)
ggplot(my_data,
aes(x = group, y = MIC, colour = SIR)) +
geom_jitter(width = 0.2, size = 2) +
geom_boxplot(fill = NA, colour = "grey40") +
scale_y_mic() +
scale_colour_sir() +
labs(title = "MIC Distribution and SIR Interpretation",
x = "Sample Groups",
y = "MIC (mg/L)")
This plot provides an intuitive way to assess susceptibility patterns across different groups while incorporating clinical breakpoints.
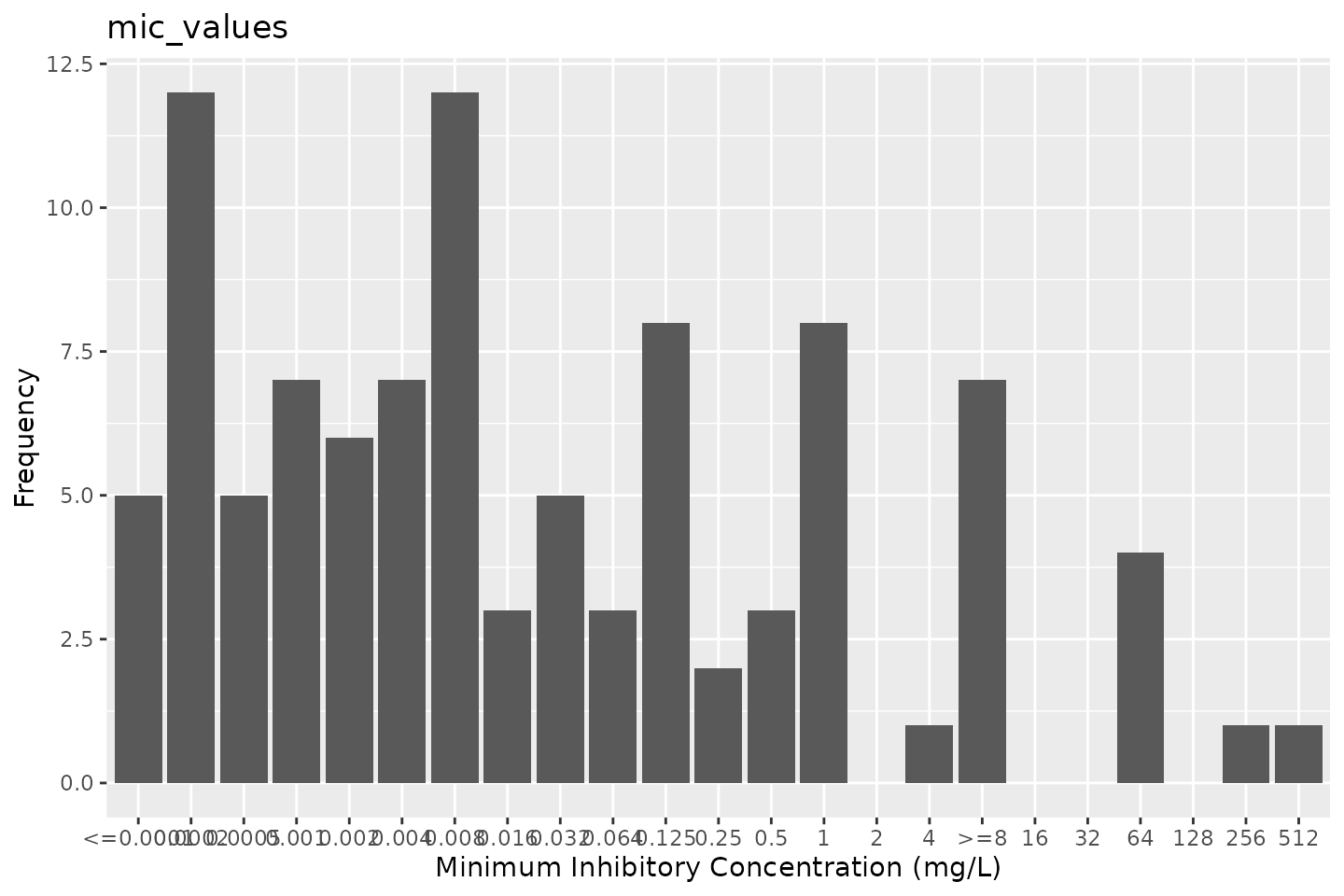
For a more straightforward and less manual approach, ggplot2’s
function
autoplot()
has been extended by this package to directly plot MIC and disk
diffusion values:
autoplot(mic_values)
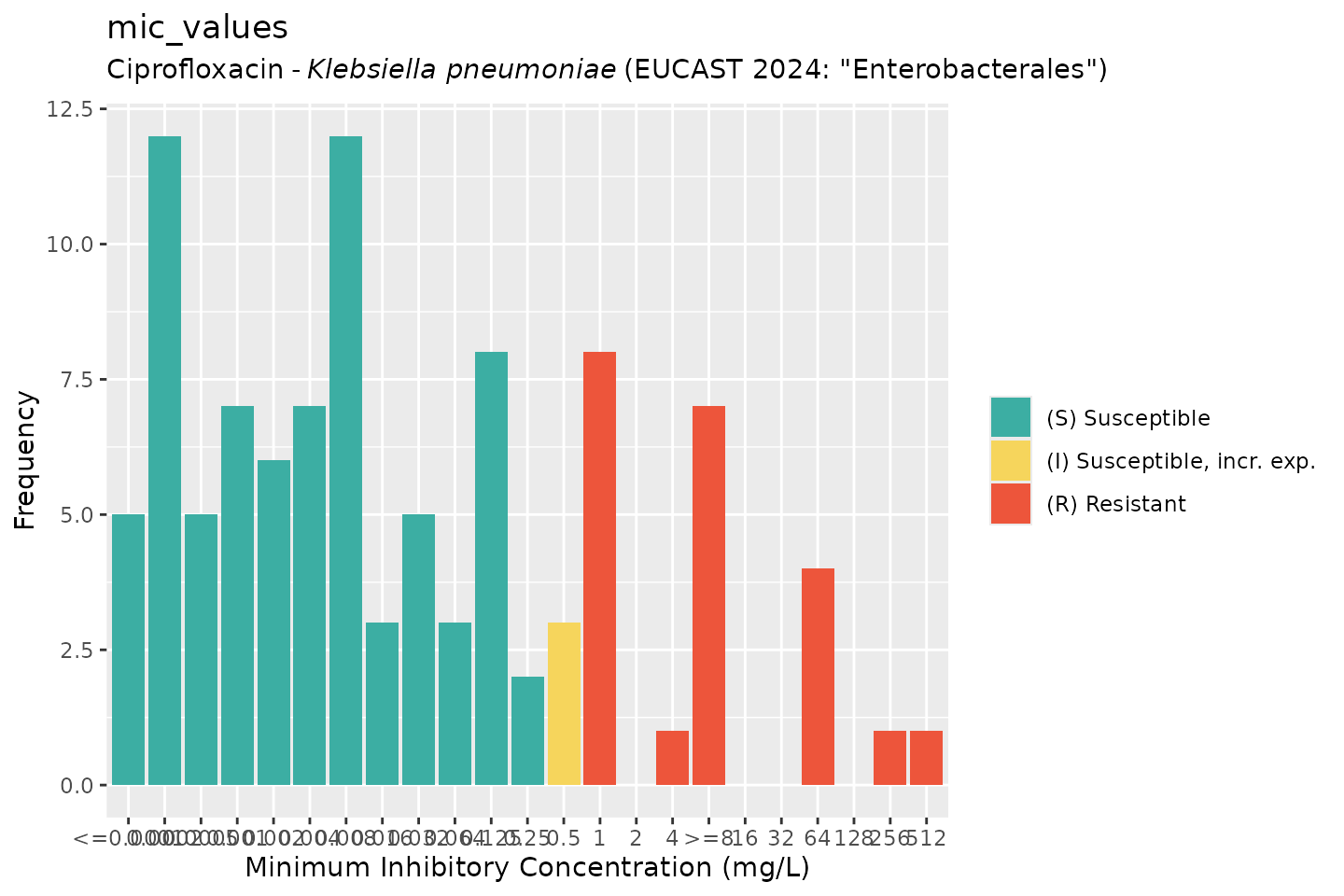
# by providing `mo` and `ab`, colours will indicate the SIR interpretation:
autoplot(mic_values, mo = "K. pneumoniae", ab = "cipro", guideline = "EUCAST 2024")
Author: Dr. Matthijs Berends, 23rd Feb 2025